Conversions Solution Stoichiometry

Conversions Solution Stoichiometry Determine amounts of reactants or products in aqueous solutions. as we learned previously, double replacement reactions involve the reaction between ionic compounds in solution and, in the course of the reaction, the ions in the two reacting compounds are “switched” (they replace each other). Knowing the volume and concentration of a solution containing one reactant, we can determine how much of another solution of another reactant will be needed using the balanced chemical equation.

Conversions Solution Stoichiometry Convert from volume of a solution of one substance to volume of a solution of another substance in a chemical reaction. the second pathway we will look at is starting with volume (ml or l) of one chemical in an equation and ending with volume (ml or l) of another. see the highlighted portion below. here's an example of how it will work. For each of the given stoichiometry conversions, identify what information is required in the solution – molar mass values, coefficients from the equation, both of these. Learn how to calculate stoichiometry in solution reactions using concentration and volume for accurate determination of reactants and products. Write a balanced chemical equation. dissociate all strong electrolytes. cross out anything that remains unchanged from the left side to the right side of the equation. write the net ionic equation with the species that remain. nacl(aq) agno3(aq) > agcl(s) nano3(aq) when dissolved in water. you must remember these.
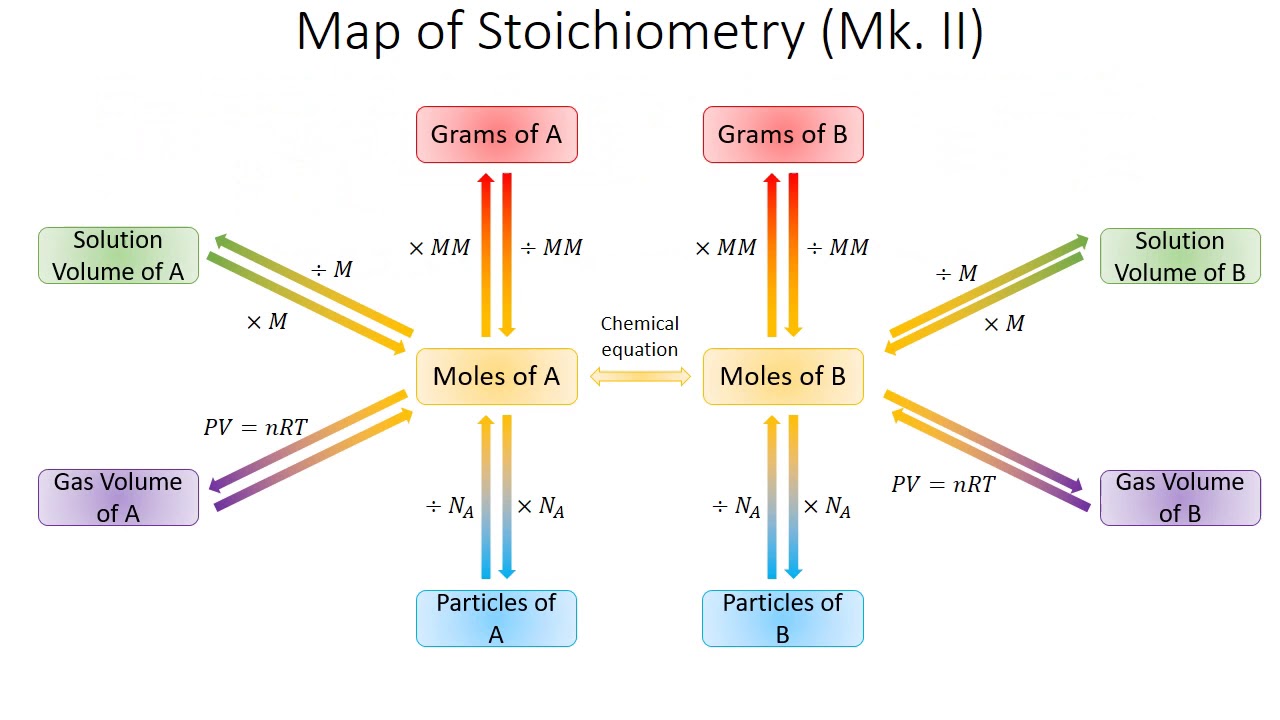
Solution Stoichiometry Chemistry Libretexts 59 Off Learn how to calculate stoichiometry in solution reactions using concentration and volume for accurate determination of reactants and products. Write a balanced chemical equation. dissociate all strong electrolytes. cross out anything that remains unchanged from the left side to the right side of the equation. write the net ionic equation with the species that remain. nacl(aq) agno3(aq) > agcl(s) nano3(aq) when dissolved in water. you must remember these. Once the moles have been determined, we can then use the balanced chemical equation to solve stoichiometry problems that involve solutions. example 1. if 100.00 ml of 0.475 m nicl 2 is reacted with 100.00 ml of 0.325 m na 2 s, how many grams of solid nis are formed? below is the balanced chemical equation. A conversion factor is a ratio or fraction which represents the relationship between two different units. a conversion factor is always equal to 1. it is extremely important to keep track of your units and keep everything neat when using conversion factors. Solution: a) first step: i2(s) cl2(g) → 2 icl(s) second step: icl(s) cl2(g) → icl3(s) b) multiply the coefficients of the second equation by 2, so that icl(s), an intermediate product, can be eliminated from the overall equation. List the steps for converting 44 g co 2 to moles. we explored stoichiometric calculations —their meaning, steps, key error checks, and real life applications.
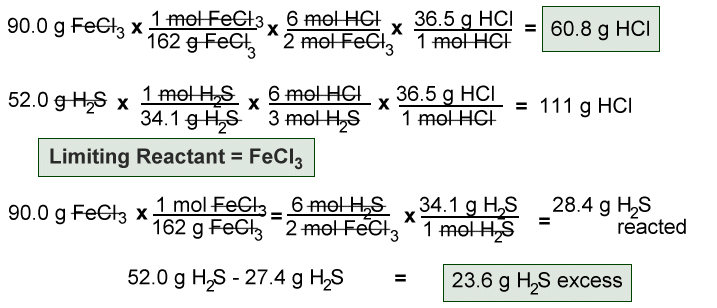
Limiting Reactant Solution Stoichiometry Once the moles have been determined, we can then use the balanced chemical equation to solve stoichiometry problems that involve solutions. example 1. if 100.00 ml of 0.475 m nicl 2 is reacted with 100.00 ml of 0.325 m na 2 s, how many grams of solid nis are formed? below is the balanced chemical equation. A conversion factor is a ratio or fraction which represents the relationship between two different units. a conversion factor is always equal to 1. it is extremely important to keep track of your units and keep everything neat when using conversion factors. Solution: a) first step: i2(s) cl2(g) → 2 icl(s) second step: icl(s) cl2(g) → icl3(s) b) multiply the coefficients of the second equation by 2, so that icl(s), an intermediate product, can be eliminated from the overall equation. List the steps for converting 44 g co 2 to moles. we explored stoichiometric calculations —their meaning, steps, key error checks, and real life applications.
Comments are closed.