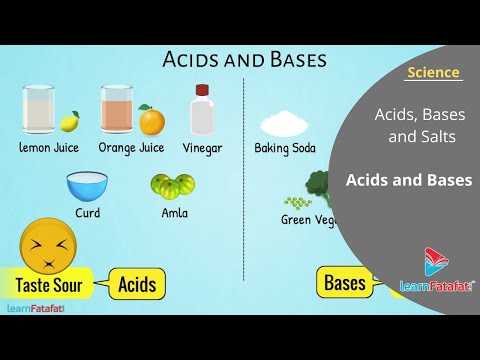
The subject of what are acids and bases encompasses a wide range of important elements. 6.1: What is an Acid and a Base? - Chemistry LibreTexts. Acids and bases neutralize each other. Hydrochloric acid is found in the stomach that helps digestion. Excess hydrochloric acid may cause acid burns—antacids like milk of magnesia are bases that help by neutralizing excess acid in the stomach.
What are acids and bases? An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and promotes certain chemical reactions (acid catalysis). Bases: What’s the Difference?. It's important to note that, while most discussions of acids and bases focus on aqueous (water-based) solutions, acid-base chemistry does not stop at water’s edge.
In non-aqueous environments—like organic solvents—acids and bases behave differently. Acid-Base Chemistry - Science Notes and Projects. Learn the basics of acid-base chemistry. Get the definitions and examples of acids and bases and learn about the neutralization reaction. Acids and Bases: Definition, Types, Properties, Safety_2025.

In chemistry, acids and bases are two essential groups of substances that have clearly different chemical properties. An acid releases hydrogen ions (H⁺) when dissolved in a solution, whereas a base either accepts hydrogen ions or releases hydroxide ions (OH⁻). This perspective suggests that, acids and Bases - GeeksforGeeks. Arrhenius's concept of acid and bases is the basic concept which explains the concept of acid and base. According to Arrhenius, "Acids are the substance that on dissolving in water releases H+ ions."
Properties of Acids and Bases: Characteristics and Everyday Examples. Furthermore, acids and bases play a vital role in chemistry, with distinct properties such as taste, feel, reactivity, and interaction with indicators. It's important to note that, this lesson explores the characteristic properties of acids and bases, helping students connect real-world observations to chemical behavior. Acid vs Base - Difference and Comparison | Diffen.

Bases are the chemical opposite of acids. Similarly, acids are defined as compounds that donate a hydrogen ion (H +) to another compound (called a base). Acids and Bases Explained: Properties, Examples & Uses - Vedantu.
Learn about acids and bases, their properties, real-life examples, and everyday uses—perfect for students and science learners. Definitions of Acids and Bases, and the Role of Water. These definitions tie the theory of acids and bases to a simple laboratory test for acids and bases. To decide whether a compound is an acid or a base we dissolve it in water and test the solution to see whether the H + or OH - ion concentration has increased.


📝 Summary
The key takeaways from this article on what are acids and bases reveal the value of knowing these concepts. Through implementing these insights, one can achieve better results.
Thanks for reading this article on what are acids and bases. Keep learning and keep discovering!