Whats A Part Its How To Get The Right Dilution Ratio

02 1 Dilution Ratio Calculation Pdf How do you calculate the dilution ratio? the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. our tool has a built in volume conversion, so you will be able to perform your calculations using any units you want!. Use this dilution ratio calculator to quickly determine the right mix of concentrate and water for any solution. simply enter the desired dilution ratio, final volume, or available concentrate, and get instant, accurate measurements in ml, liters, ounces, or gallons.

Dilution Techniques I Single Dilutions Pdf Concentration Ratio To calculate a dilution ratio, divide the total parts of water by the total parts of the solution or substance. how to calculate liquid dilution ratio? the following example problems outline how to calculate liquid dilution ratio. first, determine the total parts water. the total parts water is given as: 50. Learn how to easily calculate dilution ratios for 32oz bottles, 128oz gallons, or any other container size. dilution reference chart included!. If you know the right dilution ratio, you can make a solution with the right strength without wasting materials or putting safety at risk. if you see a ratio like 1:10, it means you should mix one part of concentrate with nine parts of water to make ten parts. this way, everything is the same, safe, and right. Calculating dilution ratios for detailing chemicals can be simplified using a simple formula. you can calculate the number of ounces required for the dilution by adding the two numbers in the ratio and dividing the total volume by that sum.

Dilution Ratio If you know the right dilution ratio, you can make a solution with the right strength without wasting materials or putting safety at risk. if you see a ratio like 1:10, it means you should mix one part of concentrate with nine parts of water to make ten parts. this way, everything is the same, safe, and right. Calculating dilution ratios for detailing chemicals can be simplified using a simple formula. you can calculate the number of ounces required for the dilution by adding the two numbers in the ratio and dividing the total volume by that sum. The following formulas can be used to calculate the volumes of solute (vsolute) and solvent (vsolvent) to be used: [1] where vtotal is the desired total volume, and f is the desired dilution factor number (the number in the position of f if expressed as " 1 f dilution factor " or " xf dilution "). Use the formula: dilution factor = v2 v1. this straightforward calculation provides the factor by which the original solution is diluted. a dilution factor of 2, for instance, implies the original solution is halved in concentration. this interpretation is crucial for accurate experimentation. How to use the dilution ratio calculator input the amount of concentrate you have in milliliters (ml) in the first field. for the dilution ratio, use the format of “1:3” where “1” represents the part of concentrate and “3” represents parts of the solvent you need to add. To calculate a dilution ratio, you need two pieces of information: the volume or weight of the concentrated substance (let’s call it “concentrate”), and the volume or weight of the solvent (let’s call it “solvent”). let’s start with a straightforward example: mixing concentrate with solvent in a 1:10 dilution ratio. formula time!.
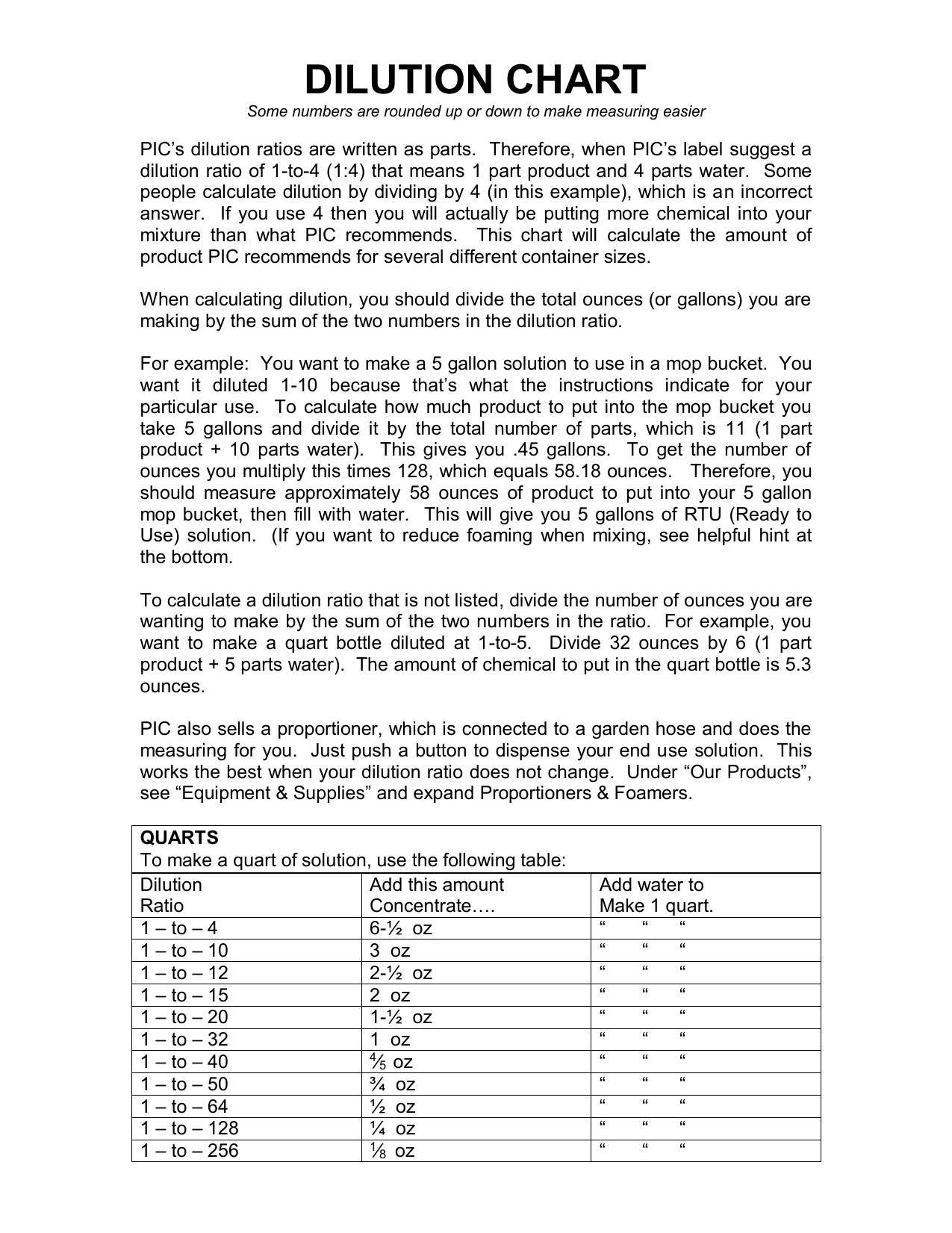
Dilution Ratio Chart A Visual Reference Of Charts Chart Master The following formulas can be used to calculate the volumes of solute (vsolute) and solvent (vsolvent) to be used: [1] where vtotal is the desired total volume, and f is the desired dilution factor number (the number in the position of f if expressed as " 1 f dilution factor " or " xf dilution "). Use the formula: dilution factor = v2 v1. this straightforward calculation provides the factor by which the original solution is diluted. a dilution factor of 2, for instance, implies the original solution is halved in concentration. this interpretation is crucial for accurate experimentation. How to use the dilution ratio calculator input the amount of concentrate you have in milliliters (ml) in the first field. for the dilution ratio, use the format of “1:3” where “1” represents the part of concentrate and “3” represents parts of the solvent you need to add. To calculate a dilution ratio, you need two pieces of information: the volume or weight of the concentrated substance (let’s call it “concentrate”), and the volume or weight of the solvent (let’s call it “solvent”). let’s start with a straightforward example: mixing concentrate with solvent in a 1:10 dilution ratio. formula time!.
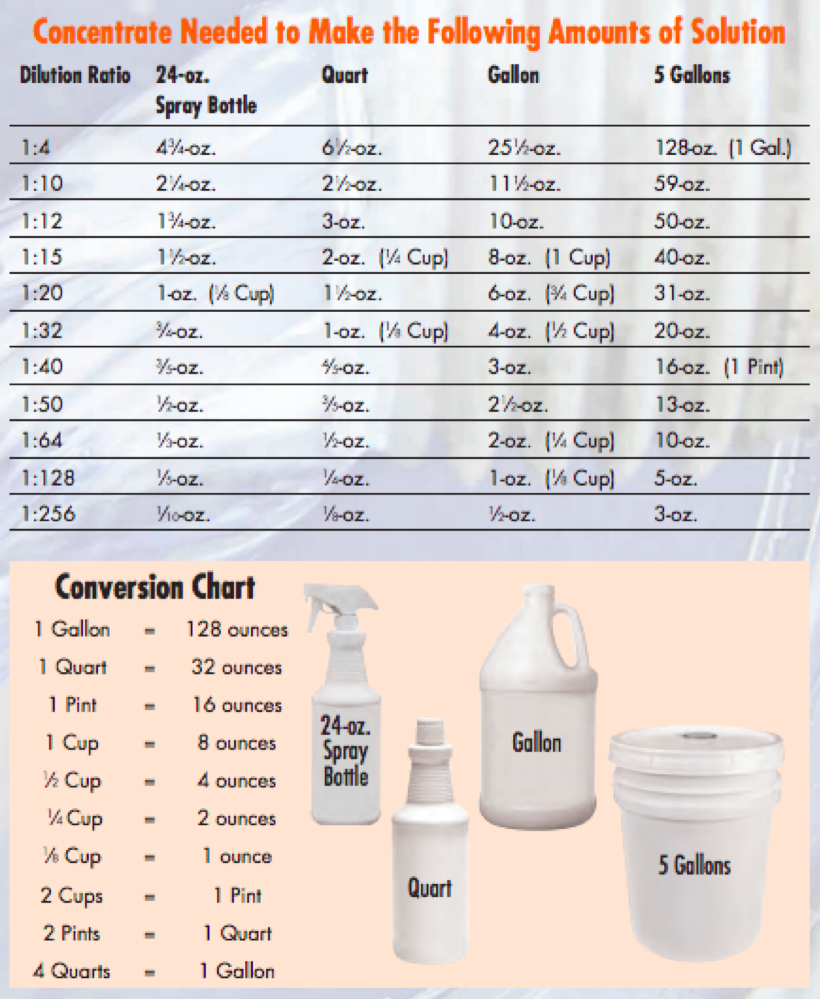
Dilution Ratio Chart A Visual Reference Of Charts Chart Master How to use the dilution ratio calculator input the amount of concentrate you have in milliliters (ml) in the first field. for the dilution ratio, use the format of “1:3” where “1” represents the part of concentrate and “3” represents parts of the solvent you need to add. To calculate a dilution ratio, you need two pieces of information: the volume or weight of the concentrated substance (let’s call it “concentrate”), and the volume or weight of the solvent (let’s call it “solvent”). let’s start with a straightforward example: mixing concentrate with solvent in a 1:10 dilution ratio. formula time!.
Comments are closed.