What Is Valency Difference Between Valency Oxidation Number
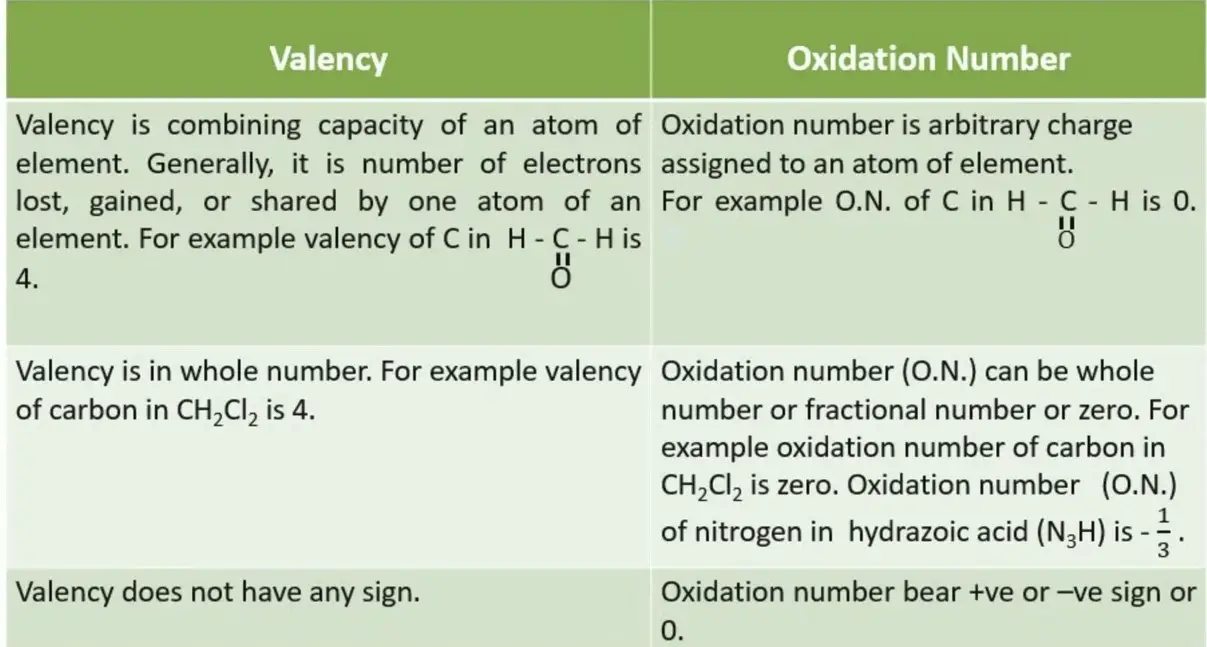
Difference Between Valency And Oxidation Number Relationship Between The main difference between valency and oxidation number is that while oxidation number refers to the number of electrons an atom can lose or gain to establish a bond with another atom, valency refers to the maximum number of electrons an atom can lose, gain, or share to become stable. Oxygen has always valency 2 2. but it has an oxidation number equal to −2 2 in the vast majority of its compounds, like water hx2o h x 2 o or cox2 c o x 2. it can also be at oxidation number −1 1 in hx2ox2 h x 2 o x 2, or at oxidation number zero in ox2 o x 2. it can be at 1 1 in the molecule ox2fx2 o x 2 f x 2, and at 2 2 in ofx2 o f x 2.
Difference Between Valency And Oxidation Number Relationship Between An element’s valence is the number of hydrogen atoms which can combine with or replace (directly or indirectly) one of the element’s atoms. oxygen, for instance, has six valence electrons but its valence is 2. Difference between rutherford and bohr model de broglie concept of dual nature of electron what are the 4 principal quantum numbers? difference between orbit and orbital with diagram state pauli’s exclusion principle and hund’s rule of maximum multiplicity e tech basic electrical quantities and their units. Oxidation state is the amount of charge on an atom which is developed in the formation of a molecule, while valency is the capacity of an atom to combine with the same or different atoms to give compounds. 2. Difference between valency and oxidation : valency is defined as the number of valence electrons in a neutral atom, whereas oxidation number is defined as the oxidation state which is due to the electronegativity difference of that atom in the molecule (after gaining or losing an electron).
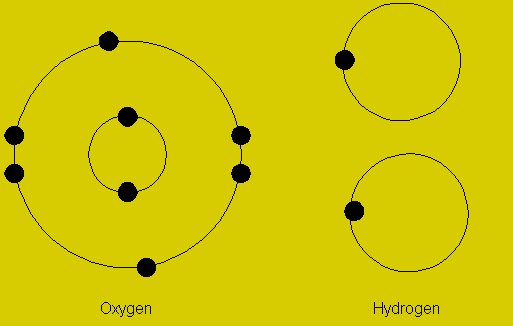
Difference Between Valency And Oxidation State Oxidation state is the amount of charge on an atom which is developed in the formation of a molecule, while valency is the capacity of an atom to combine with the same or different atoms to give compounds. 2. Difference between valency and oxidation : valency is defined as the number of valence electrons in a neutral atom, whereas oxidation number is defined as the oxidation state which is due to the electronegativity difference of that atom in the molecule (after gaining or losing an electron). The valency is the number of bonds. the degree of oxidation is the number of charges that are left on an atom once all valence bonds between different elements are replaced by an ionic bond. oxygen has two valences in the molecule h o o h.
Comments are closed.