Using The Figure Shown Below As Reference Draw The Resonance
Solved 2 23 For Each Of The Structures Below Draw The Resonance Exercise 2.6.6: draw the major resonance contributor for each of the anions below. c) fill in the blanks: the conjugated pi system in part (a) is composed of 2p orbitals containing delocalized pi electrons. exercise 2.6.7: the figure below shows how the negative formal charge on the oxygen can be delocalized to the carbon indicated. A) draw three additional resonance contributors for the carbocation below. include in your figure the appropriate curved arrows showing how one contributor is converted to the next. b) fill in the blanks: the conjugated pi system in this carbocation is composed of 2p orbitals sharing delocalized pi electrons.
Solved O 6 Draw The Resonance Structures For The Following Molecules A) draw three additional resonance contributors for the carbocation below. include in your figure the appropriate curved arrows showing how one contributor is converted to the next. b) fill in the blanks: the conjugated pi system in this carbocation is composed of 2p orbitals sharing delocalized pi electrons. answer. a). Consider the structure of a carboxylic ester as shown below. at first sight, this might look like ‘half a carbonate’, which would mean that both shown resonance structures are equal, contribute $0.5$ to the overall picture and therefore both bond orders would be $1.5$. however, this is only the case if we are talking about the carboxylate. Step 1: draw the lewis dot structure of no 3–, as shown below. the procedure is discussed in our lewis structure article. the image shows that no 3– has two oxygen atoms (o1 and o3) with three lone pairs. also, these two oxygen atoms have a 1 charge and are single bonded to the nitrogen atom. 2. introducing curved arrows, a tool for showing the movement of electrons between resonance structures. here’s the punch line: we can convert one resonance form into another by showing the movement of electrons between bonds and lone pairs (or vice versa). we just need a graphical tool to do it.
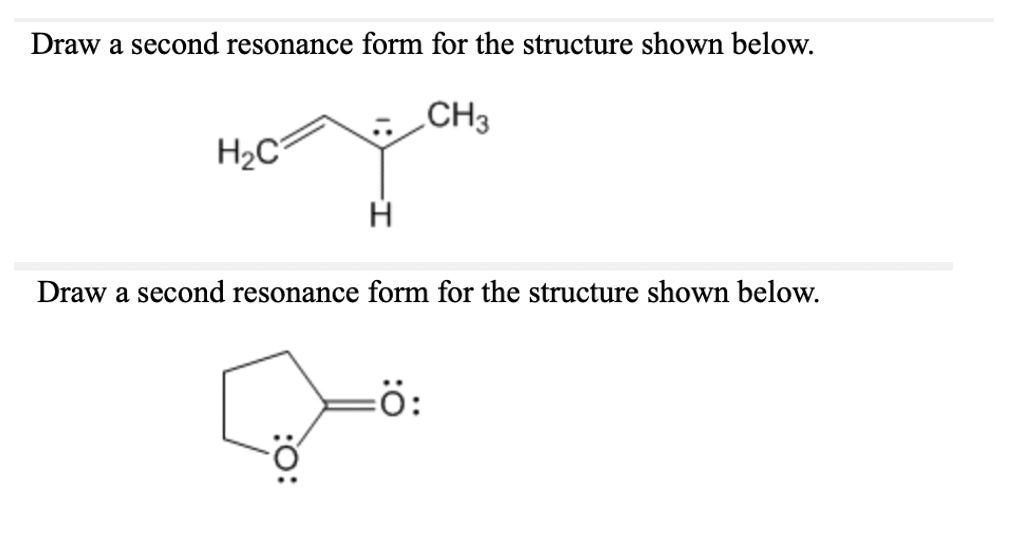
Solved Draw A Second Resonance Form For The Structure Shown Below Ch3 Step 1: draw the lewis dot structure of no 3–, as shown below. the procedure is discussed in our lewis structure article. the image shows that no 3– has two oxygen atoms (o1 and o3) with three lone pairs. also, these two oxygen atoms have a 1 charge and are single bonded to the nitrogen atom. 2. introducing curved arrows, a tool for showing the movement of electrons between resonance structures. here’s the punch line: we can convert one resonance form into another by showing the movement of electrons between bonds and lone pairs (or vice versa). we just need a graphical tool to do it. Since the resonance hybrid is a combination of the two resonance structures we see that we have a 1 2 formal charge on each of the oxygens and the bonds between the n and os is 1 and 1 2 or 1.5 (or 3 2). we should remember that a molecule described as a resonance hybrid never possesses an electronic structure described by either resonance form. 1. draw the resonance contributors that correspond to the curved, two electron movement arrows in the resonance expressions below. 2. in each resonance expression, draw curved two electron movement arrows on the left side contributor that shows how we get to the right side contributor. be sure to include formal charges.
Solved Draw A Second Resonance Form For The Structure Shown Below H Since the resonance hybrid is a combination of the two resonance structures we see that we have a 1 2 formal charge on each of the oxygens and the bonds between the n and os is 1 and 1 2 or 1.5 (or 3 2). we should remember that a molecule described as a resonance hybrid never possesses an electronic structure described by either resonance form. 1. draw the resonance contributors that correspond to the curved, two electron movement arrows in the resonance expressions below. 2. in each resonance expression, draw curved two electron movement arrows on the left side contributor that shows how we get to the right side contributor. be sure to include formal charges.
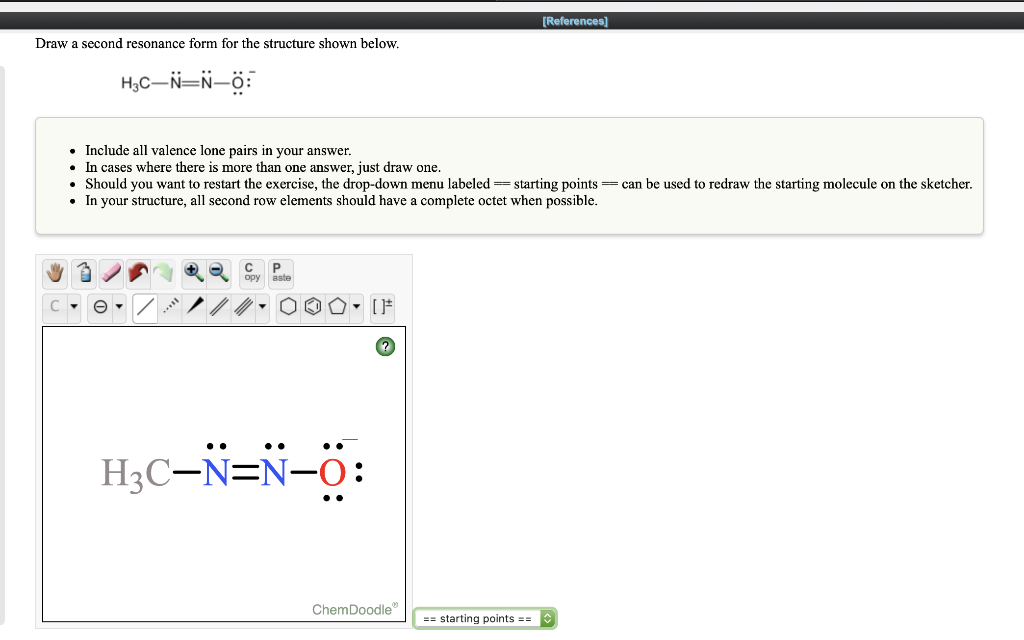
Solved References Draw A Second Resonance Form For The Chegg

Comments are closed.