Stoichiometry Using Molarity To Find Solute Moles And Solution Volume
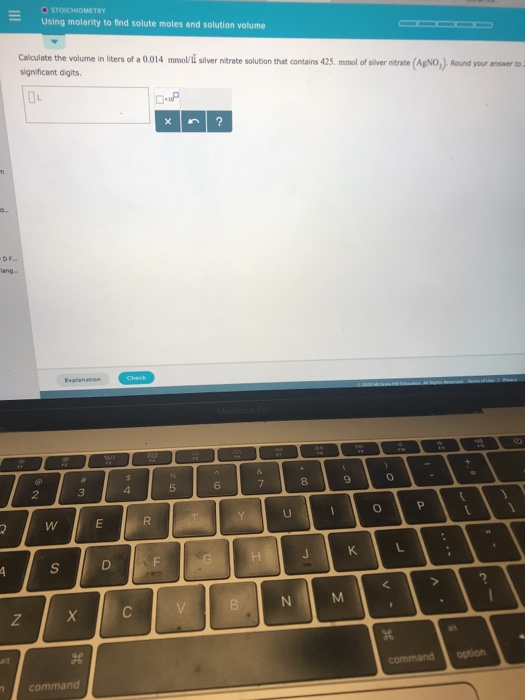
Solved Stoichiometry Using Molarity To Find Solute Moles And Chegg Because these reactions occur in aqueous solution, we can use the concept of molarity to directly calculate the number of moles of reactants or products that will be formed, and therefore their amounts (i.e. volume of solutions or mass of precipitates). This chemistry video tutorial explains how to solve solution stoichiometry problems. it discusses how to balance precipitation reactions and how to calculate the molarity or the concentration.

Solved Stoichiometry Using Molarity To Find Solute Moles And Chegg If given a molar concentration and the solution volume, the number of moles of solute is easy to calculate. once the moles have been determined, we can then use the balanced chemical equation to solve stoichiometry problems that involve solutions. Molarity (m) is a useful concentration unit for many applications in chemistry. molarity is defined as the number of moles of solute in exactly 1 litre (1 l) of the solution: example 1.4.1 – calculating molar concentrations. a 355 ml soft drink sample contains 0.133 mol of sucrose (table sugar). Knowing the volume (liters) of solution and the molarity is enough to determine the moles of solute. if the solute is a reactant, these moles can be used in limiting reacatant problems to determine the amount of product expected from the reaction. Stoichiometry allows us to work in solution by giving us the concept of solution concentration, or molarity. molarity is a unit that is often abbreviated as capital m. it is defined as the moles of a substance contained in one liter of solution.

Solved Stoichiometry Using Molarity To Find Solute Moles And Chegg Knowing the volume (liters) of solution and the molarity is enough to determine the moles of solute. if the solute is a reactant, these moles can be used in limiting reacatant problems to determine the amount of product expected from the reaction. Stoichiometry allows us to work in solution by giving us the concept of solution concentration, or molarity. molarity is a unit that is often abbreviated as capital m. it is defined as the moles of a substance contained in one liter of solution. Calculate concentrations of solutions in molarity, molality, mole fraction and percent by mass and volume. stoichiometry deals with the relative quantities of reactants and products in chemical reactions. it can be used to find the quantities of the products from given reactants in a balanced chemical reaction, as well as percent yield. Solution stoichiometry calculations involve chemical reactions taking place in solution. of the various methods of expressing solution concentration the most convenient for general laboratory use is molarity, which is defined: molarity = moles of solute liters of solution or m = nsolute. Calculate molarity of a solution and solve stoichiometry problems using solution molarities. in preceding sections, we focused on the composition of substances: samples of matter that contain only one type of element or compound. Chemists express the concentration of a solution using molarity (m), the number of moles of solute per litre of solution. by combining this with balanced chemical equations, we can perform solution stoichiometry calculations to determine how much of each substance reacts or forms in an aqueous process. in this section, you'll learn how to calculate molarity, perform dilutions, and use volume.
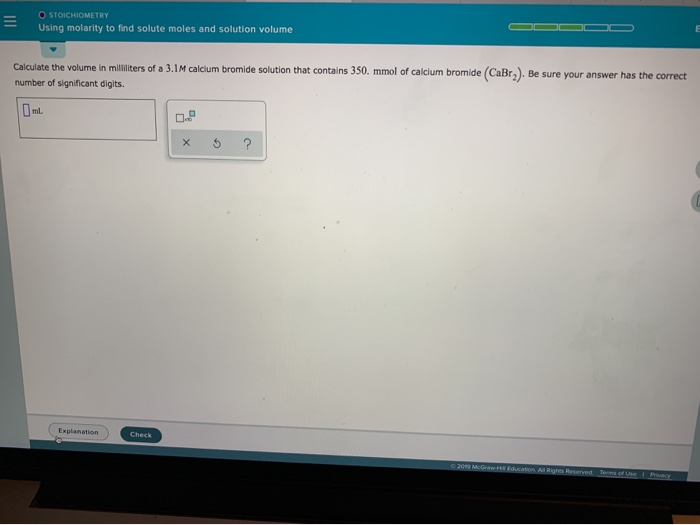
Solved O Stoichiometry Using Molarity To Find Solute Moles Chegg Calculate concentrations of solutions in molarity, molality, mole fraction and percent by mass and volume. stoichiometry deals with the relative quantities of reactants and products in chemical reactions. it can be used to find the quantities of the products from given reactants in a balanced chemical reaction, as well as percent yield. Solution stoichiometry calculations involve chemical reactions taking place in solution. of the various methods of expressing solution concentration the most convenient for general laboratory use is molarity, which is defined: molarity = moles of solute liters of solution or m = nsolute. Calculate molarity of a solution and solve stoichiometry problems using solution molarities. in preceding sections, we focused on the composition of substances: samples of matter that contain only one type of element or compound. Chemists express the concentration of a solution using molarity (m), the number of moles of solute per litre of solution. by combining this with balanced chemical equations, we can perform solution stoichiometry calculations to determine how much of each substance reacts or forms in an aqueous process. in this section, you'll learn how to calculate molarity, perform dilutions, and use volume.
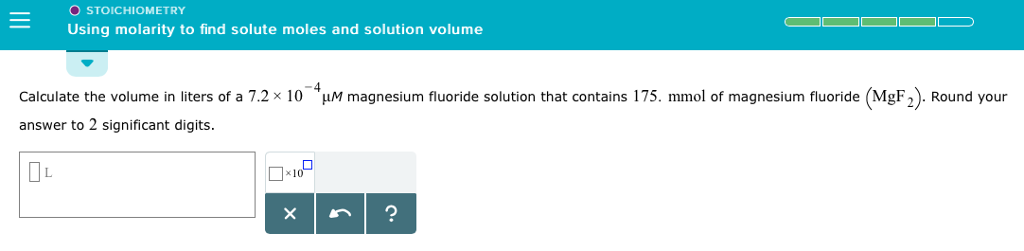
Solved O Stoichiometry Using Molarity To Find Solute Moles Chegg Calculate molarity of a solution and solve stoichiometry problems using solution molarities. in preceding sections, we focused on the composition of substances: samples of matter that contain only one type of element or compound. Chemists express the concentration of a solution using molarity (m), the number of moles of solute per litre of solution. by combining this with balanced chemical equations, we can perform solution stoichiometry calculations to determine how much of each substance reacts or forms in an aqueous process. in this section, you'll learn how to calculate molarity, perform dilutions, and use volume.
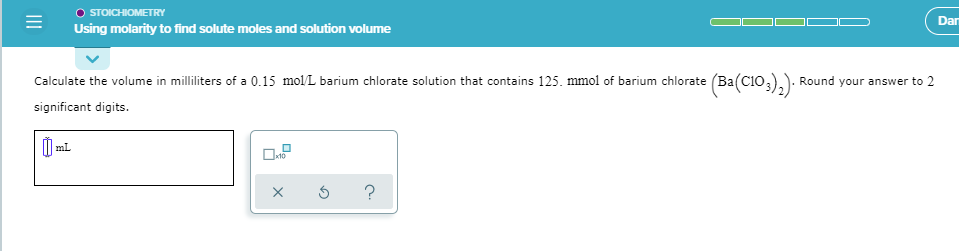
Solved Stoichiometry Using Molarity To Find Solute Moles And Chegg
Comments are closed.