Solved This Data Was Obtained By Measuring The Formation Of Chegg

Chegg Pdf There are 3 steps to solve this one. in this question, we will explore the concept of rate law. rate law is the expression in which the ra. This page discusses the importance of accurate time measurement in studying chemical reaction rates and determining rate laws through experiments with different reactant concentrations.

Solved This Data Was Obtained By Measuring The Formation Of Chegg Example #1: calculate the enthalpy for this reaction: given the following thermochemical equations: 1) determine what we must do to the three given equations to get our target equation: c) third eq: do nothing. we need one h 2 on the reactant side and that's what we have. 2) rewrite all three equations with changes applied:. According to the general law, keq for reaction 3 takes the following form. our purpose in the experiment will be to evaluate keq for the reaction by determining the equilibrium concentrations of the four species in equation 4 in several solutions made up in different ways. Starting with known amounts of iron (iii) and thiocyanate, and measuring the amount of fescn2 ion formed at equilibrium, one can calculate the equilibrium amounts of iron (iii) and thiocyanate ions. The initial reaction rates were obtained measuring the initial slope of the progress curve of product formation. the data in the below were obtained: what is he vmax for this enzyme reaction? what is the km for the substrate?.
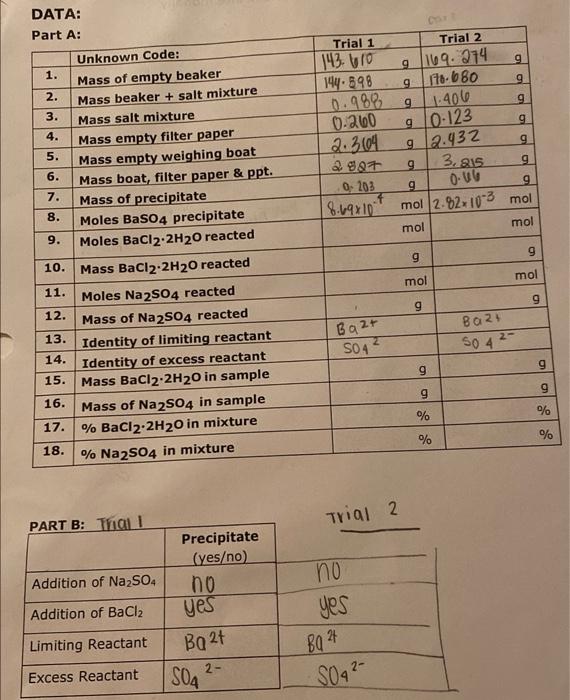
Solved Data Chegg Starting with known amounts of iron (iii) and thiocyanate, and measuring the amount of fescn2 ion formed at equilibrium, one can calculate the equilibrium amounts of iron (iii) and thiocyanate ions. The initial reaction rates were obtained measuring the initial slope of the progress curve of product formation. the data in the below were obtained: what is he vmax for this enzyme reaction? what is the km for the substrate?. How do you find the concentration of an unknown solution? can also be found by the slope of beer's law curve and the equation y=mx b. the direct relationship between absorbance and concentration for a solution. direct relationship. what is measured to find absorbance of each solution?. Calculate the average rate of reaction during the first minute and during the second minute. why are these two rates not equal? in the reaction a → products, 4.50 minutes after the reaction is started, [a] = 0.587m. the rate of reaction at this point is rate = −Δ[a] Δt = 2.1 ×10−2 mmin−1. Knowing the initial concentrations of reactants and the measured equilibrium molarity of fescn2 , you can complete the initial change equilibrium (ice) table and determine kc as we have done in several example and homework problems. we use a spectrophotometer to determine [fescn2 ] in the equilibrium mixtures. Answers: (a) 1, (b) m–1 s–1 the initial rate of a reaction a b c was measured for several different starting concentrations of a and b, and the results are as follows: using these data, determine (a) the rate law for the reaction, (b) the rate constant, (c) the rate of the reaction when [a] = 0.050 m and [b] = 0.100 m.
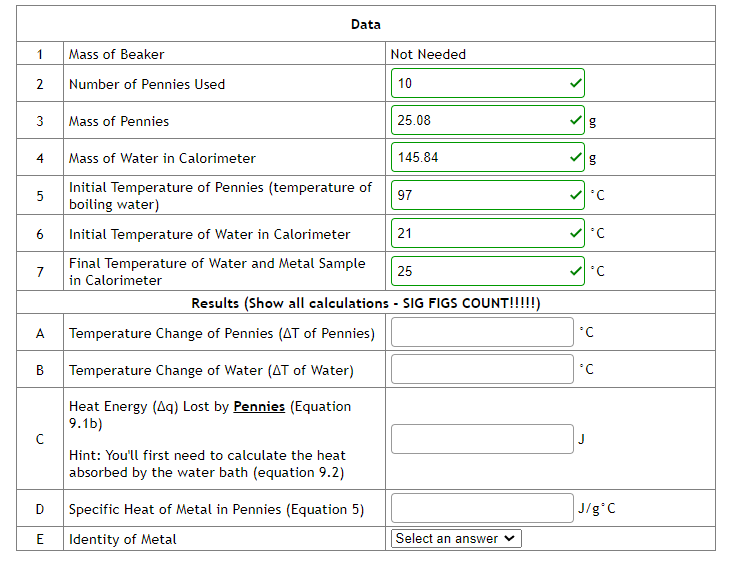
Solved Data Chegg How do you find the concentration of an unknown solution? can also be found by the slope of beer's law curve and the equation y=mx b. the direct relationship between absorbance and concentration for a solution. direct relationship. what is measured to find absorbance of each solution?. Calculate the average rate of reaction during the first minute and during the second minute. why are these two rates not equal? in the reaction a → products, 4.50 minutes after the reaction is started, [a] = 0.587m. the rate of reaction at this point is rate = −Δ[a] Δt = 2.1 ×10−2 mmin−1. Knowing the initial concentrations of reactants and the measured equilibrium molarity of fescn2 , you can complete the initial change equilibrium (ice) table and determine kc as we have done in several example and homework problems. we use a spectrophotometer to determine [fescn2 ] in the equilibrium mixtures. Answers: (a) 1, (b) m–1 s–1 the initial rate of a reaction a b c was measured for several different starting concentrations of a and b, and the results are as follows: using these data, determine (a) the rate law for the reaction, (b) the rate constant, (c) the rate of the reaction when [a] = 0.050 m and [b] = 0.100 m.
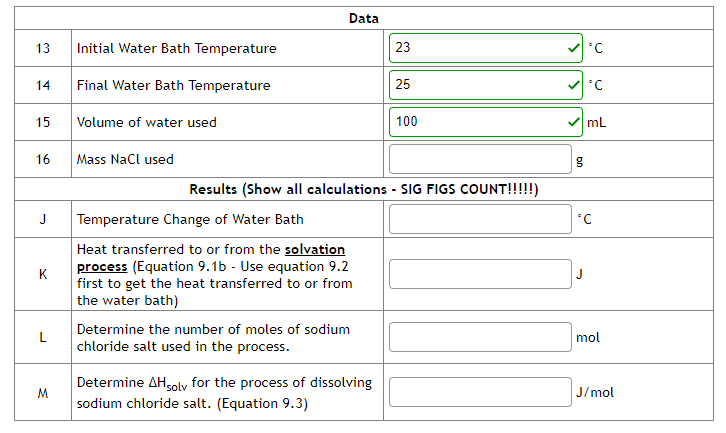
Solved Data Chegg Knowing the initial concentrations of reactants and the measured equilibrium molarity of fescn2 , you can complete the initial change equilibrium (ice) table and determine kc as we have done in several example and homework problems. we use a spectrophotometer to determine [fescn2 ] in the equilibrium mixtures. Answers: (a) 1, (b) m–1 s–1 the initial rate of a reaction a b c was measured for several different starting concentrations of a and b, and the results are as follows: using these data, determine (a) the rate law for the reaction, (b) the rate constant, (c) the rate of the reaction when [a] = 0.050 m and [b] = 0.100 m.
Comments are closed.