Solved The Percent Yield Is Calculated As Follows Percent Chegg

Solved The Percent Yield Is Calculated As Follows ï Percent Chegg There are 2 steps to solve this one. the percent yield is calculated as follows: percent yield (%)= theoretical yield actual yield ×100% the theoretical yield is the mass of product theoretically created as determined by the stoichiometry calculations. Learn about the percent yield of chemical reactions. the practice problems will address finding the percent yield from a single reactant, from two reactants considering the limiting reactant and determining the amounts of reactants needed at a given percent yield.
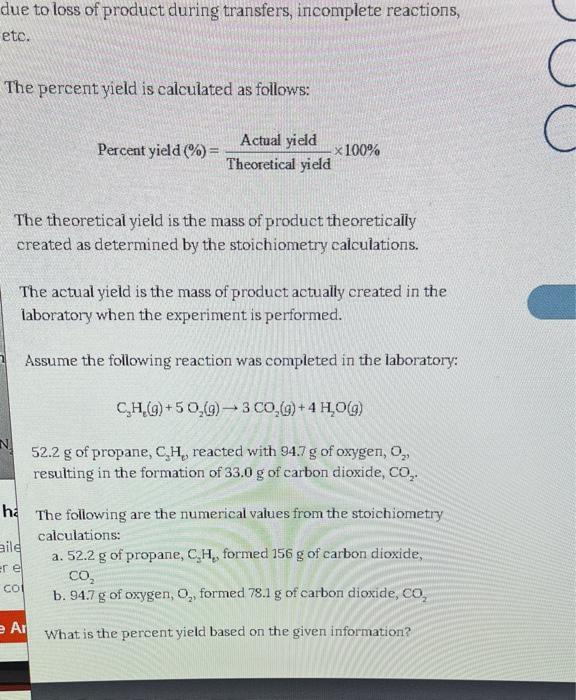
Solved The Percent Yield Is Calculated As Follows Percent Chegg The percent yield is calculated as follows: percent yield (% )= actualyield theoreticalyield * 100% the theoretical yield is the mass of product theoretically created as determined by the stoichiometry calculations. Learn how to calculate percent yield in chemistry with this easy, step by step tutorial! we’ll explain the percent yield formula, solve real examples, and walk you through practice problems with. Take your experimental yield and divide it by the theoretical yield. did we solve your problem today? are you carrying out a reaction? find your reaction efficiency with our percent yield calculator. 1. write the given and needed quantities 2. write a plan to calculate the theoretical yield and the percent yield 3. write the molar mass for the reactant and the mole mole factor from the balanced equation 4. solve for the percent yield ratio by dividing the actual yield (given) by the theoretical yield x 100.

Solved Based Upon The Percent Yield You Calculated What Is Chegg Take your experimental yield and divide it by the theoretical yield. did we solve your problem today? are you carrying out a reaction? find your reaction efficiency with our percent yield calculator. 1. write the given and needed quantities 2. write a plan to calculate the theoretical yield and the percent yield 3. write the molar mass for the reactant and the mole mole factor from the balanced equation 4. solve for the percent yield ratio by dividing the actual yield (given) by the theoretical yield x 100. To calculate percent yield in practical scenarios, follow these steps: determine the theoretical yield: use stoichiometry to calculate the maximum possible amount of product from the given reactants. measure the actual yield: experimentally obtain and weigh the product formed. Enhanced with ai, our expert help has broken down your problem into an easy to learn solution you can count on. here’s the best way to solve it. the percent yield in chemistry is calculated using the formula: $$ \text {percent yield} = \left ( \frac {\text {actual yield}} {\text {theoretical yield}} \right) \times 100\% $$. Step 1: write the balanced chemical equation. step 2: determine actual and theoretical yield. actual is given, theoretical is calculated: h 2? 4. iron pyrites (fes 2) reacts with oxygen according. 1. the electrolysis of water forms h to the following equation: 2 and o 2. We have an expert written solution to this problem! what contributes to a percent yield that is greater than 100%? what contributes to a percent yield that is less than 100%? study with quizlet and memorize flashcards containing terms like actual yield, percent yield, theoretical yield and more.
Solved What Is The Percent Yield For This Reaction When The Chegg To calculate percent yield in practical scenarios, follow these steps: determine the theoretical yield: use stoichiometry to calculate the maximum possible amount of product from the given reactants. measure the actual yield: experimentally obtain and weigh the product formed. Enhanced with ai, our expert help has broken down your problem into an easy to learn solution you can count on. here’s the best way to solve it. the percent yield in chemistry is calculated using the formula: $$ \text {percent yield} = \left ( \frac {\text {actual yield}} {\text {theoretical yield}} \right) \times 100\% $$. Step 1: write the balanced chemical equation. step 2: determine actual and theoretical yield. actual is given, theoretical is calculated: h 2? 4. iron pyrites (fes 2) reacts with oxygen according. 1. the electrolysis of water forms h to the following equation: 2 and o 2. We have an expert written solution to this problem! what contributes to a percent yield that is greater than 100%? what contributes to a percent yield that is less than 100%? study with quizlet and memorize flashcards containing terms like actual yield, percent yield, theoretical yield and more.

Solved Calculations 12 Pts Calculate The Percent Yield Of Chegg Step 1: write the balanced chemical equation. step 2: determine actual and theoretical yield. actual is given, theoretical is calculated: h 2? 4. iron pyrites (fes 2) reacts with oxygen according. 1. the electrolysis of water forms h to the following equation: 2 and o 2. We have an expert written solution to this problem! what contributes to a percent yield that is greater than 100%? what contributes to a percent yield that is less than 100%? study with quizlet and memorize flashcards containing terms like actual yield, percent yield, theoretical yield and more.
Comments are closed.