Solved Rank Alpha Particles Beta Particles Positrons And Chegg

Solved Rank Alpha Particles Beta Particles Positrons And Chegg There are 2 steps to solve this one. rank alpha particles, beta particles, positrons, and gamma rays in terms of decreasing ionizing power. Alpha particles have the highest ionizing power due to their large mass and charge, followed by beta particles and positrons which have identical ionizing powers but can be differentiated based on their charges, with gamma rays having the least ionizing power as they are neutral and have no mass.

Solved Rank Alpha Particles Beta Particles Positrons And Chegg Rank alpha particles, beta particles, positrons, and gamma rays in terms of: (a) increasing ionizing power; (b) increasing penetrating power. textbook answer. Gamma rays have the lowest ionizing power, followed by positrons, then beta particles, and finally alpha particles, which have the highest ionizing power. in short, the order is gamma rays, positrons, beta particles, and alpha particles as they interact with matter. Study with quizlet and memorize flashcards containing terms like alpha particles, beta particles, u000b positrons, and gamma radiation, alpha particle, gamma rays and more. All of the above are true. an alpha particle is a helium 2 ion. beta decay occurs when a neutron changes into a proton while emitting an electron. positrons are similar in ionizing power and penetrating power to beta particles. a positron is the antiparticle of the electron.

Solved Part B Rank Alpha Particles Beta Particles Posit Chegg Study with quizlet and memorize flashcards containing terms like alpha particles, beta particles, u000b positrons, and gamma radiation, alpha particle, gamma rays and more. All of the above are true. an alpha particle is a helium 2 ion. beta decay occurs when a neutron changes into a proton while emitting an electron. positrons are similar in ionizing power and penetrating power to beta particles. a positron is the antiparticle of the electron. Question answered step by step rank alpha particles, beta particles, positrons, and gamma rays in terms of: (a) increasing ionizing power; (b) increasing penetrating power. Alpha particles are massive and carry a 2 charge, beta particles and positrons have a much lower mass and carry a charge of 1 and 1 respectively, while gamma rays are neutral. ionizing power refers to the ability of radiation to dislodge electrons from atoms, thereby creating ions. Radiation may take the form of alpha (α) (α) and beta (β) (β) particles, positrons (β ), (β ), or pure energy such as gamma (γ) (γ) rays. There are 2 steps to solve this one. radiation ionization with sufficient energy not the question you’re looking for? post any question and get expert help quickly.
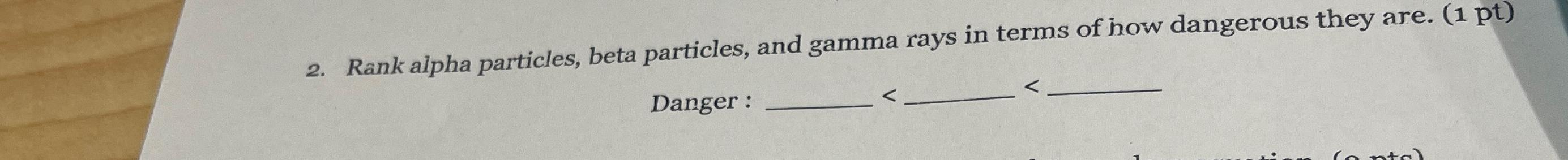
Solved Rank Alpha Particles Beta Particles And Gamma Rays Chegg Question answered step by step rank alpha particles, beta particles, positrons, and gamma rays in terms of: (a) increasing ionizing power; (b) increasing penetrating power. Alpha particles are massive and carry a 2 charge, beta particles and positrons have a much lower mass and carry a charge of 1 and 1 respectively, while gamma rays are neutral. ionizing power refers to the ability of radiation to dislodge electrons from atoms, thereby creating ions. Radiation may take the form of alpha (α) (α) and beta (β) (β) particles, positrons (β ), (β ), or pure energy such as gamma (γ) (γ) rays. There are 2 steps to solve this one. radiation ionization with sufficient energy not the question you’re looking for? post any question and get expert help quickly.
Comments are closed.