Solved Question 35 Given The Initial Concentrations Below Chegg

Solved Question 35 Given The Initial Concentrations Below Chegg Here’s the best way to solve it. que 35) the reaction quotient, q = 0, is zero since the initial concentration of the product is zero. the rxn then moves to the product …. In the following problems, you will be given initial concentrations as well as one equilibrium concentration. you have to use stoichiometry to figure out the other equilibrium concentrations from the data given.

Solved Question 4 The Initial Concentrations Or Pressures Of Chegg Given multiple mathematical solutions to x, determine the physically reasonable solution. based on k and initial concentrations, determine if x is negligible within ~5% error. for a more complex example, the quadratic equation may need to be solved to find x. The initial concentrations are: [i 2] = 0.0030 m, [h 2] = 0.0050 m, [hi] = 0.030 m. calculate the equilibrium concentrations of all species. for this problem we are given initial concentrations of both reactants and product. The initial concentrations or pressures of reactants and products are given for each of the following systems. calculate the reaction quotient and determine the direction in which each system will proceed to reach equilibrium. Plan using the law of mass action, we write each expression as a quotient having the product concentration terms in the numerator and the reactant concentration terms in the denominator. each concentration term is raised to the power of its coefficient in the balanced chemical equation. kc = 9.60 at 300 c.
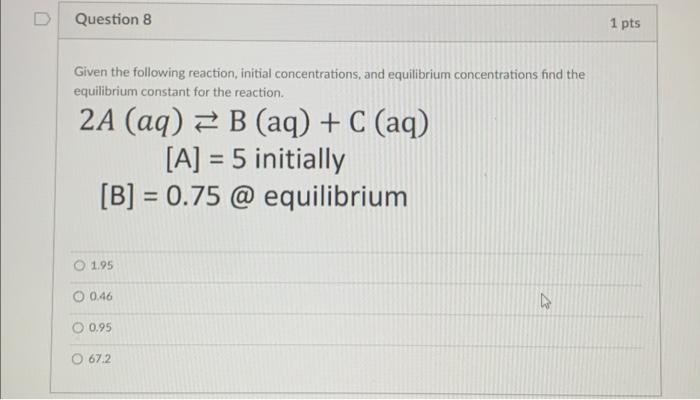
Solved Given The Following Reaction Initial Concentrations Chegg The initial concentrations or pressures of reactants and products are given for each of the following systems. calculate the reaction quotient and determine the direction in which each system will proceed to reach equilibrium. Plan using the law of mass action, we write each expression as a quotient having the product concentration terms in the numerator and the reactant concentration terms in the denominator. each concentration term is raised to the power of its coefficient in the balanced chemical equation. kc = 9.60 at 300 c. To find the equilibrium concentrations for a, b, and c, start by writing the equilibrium expression for the given chemical reaction, a (g) b (g) ↔ 2 c (g), in terms of the equilibrium constant (k c). What are the equilibrium concentrations of p cl5 p c l 5, p cl3 p c l 3, and cl2 c l 2 in a mixture that initially contained only p cl5 p c l 5 at a concentration of 1.00 m m. use the stepwise process described earlier. identify the direction in which the reaction will proceed to reach equilibrium. There are a few steps that need to be carried out to find the equilibrium concentration of a chemical reaction. the steps are as below. the second step is to convert the concentration of the products and the reactants in terms of their molarity. Solving k sp problems ii: calculating k sp when . . . . what is the minimum ph required for precipitation? the reaction quotient: will a precipitate form?.
Comments are closed.