Solved Part B Rank Alpha Particles Beta Particles Posit Chegg

Solved Rank Alpha Particles Beta Particles Positrons And Chegg There are 2 steps to solve this one. part b rank alpha particles, beta particles, positrons, and gamma rays in terms of increasing penetrating power. rank from largest to smallest penetrating power. to rank items as equivalent, overlap them. largest smalest the correct ranking cannot be determined. Alpha particles have the highest ionizing power due to their large mass and charge, followed by beta particles and positrons which have identical ionizing powers but can be differentiated based on their charges, with gamma rays having the least ionizing power as they are neutral and have no mass.
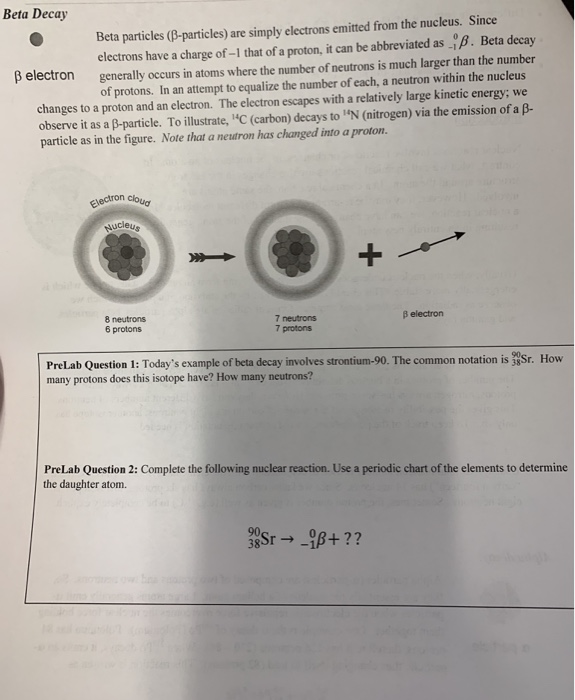
Solved Beta Decay Beta Particles B Particles Are Simply Chegg Why are alpha particles, beta particles, and gamma rays considered forms of nuclear radiation?. Beta particles: beta particles, which are either electrons or positrons, have higher ionizing power compared to positrons. they have more mass and charge, allowing for stronger interactions with matter. Rank alpha particles, beta particles, positrons, and gamma rays in terms of: (a) increasing ionizing power; (b) increasing penetrating power. textbook answer. These particles, called nuclear radiation, occur in three different forms: alpha (αalpha) particles, beta (βbeta) particles, and gamma (γgamma) rays. alpha particles are helium nuclei, with two neutrons and two protons. beta particles are high energy electrons.
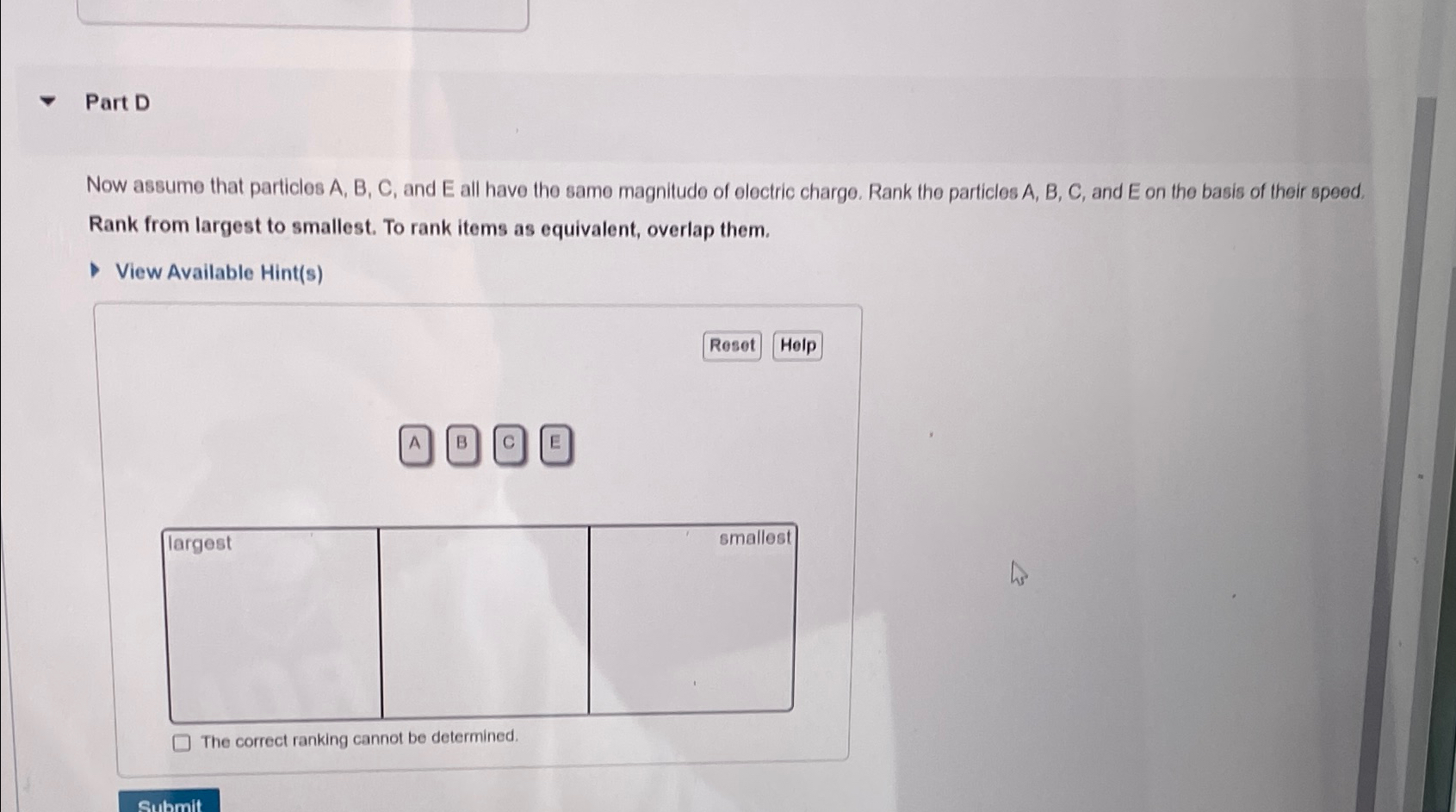
Part Dnow Assume That Particles A ï B ï C ï And E All Chegg Rank alpha particles, beta particles, positrons, and gamma rays in terms of: (a) increasing ionizing power; (b) increasing penetrating power. textbook answer. These particles, called nuclear radiation, occur in three different forms: alpha (αalpha) particles, beta (βbeta) particles, and gamma (γgamma) rays. alpha particles are helium nuclei, with two neutrons and two protons. beta particles are high energy electrons. Beta particles are much smaller than alpha particles and carry a single negative charge. they are less ionizing than alpha particles and thus have greater penetration power. gamma rays are electromagnetic radiation, not particles. they have no charge and no mass, making them the least ionizing and possessing the highest penetration power. Question answered step by step rank alpha particles, beta particles, positrons, and gamma rays in terms of: (a) increasing ionizing power; (b) increasing penetrating power. These particles, called nuclear radiation, occur in three different forms: alpha (α alpha) particles, beta (βbeta) particles, and gamma (γ gamma) rays. alpha particles are helium nuclei, with two neutrons and two protons. Which of the following statements accurately describes alpha particles in terms of charge and mass? a. alpha particles are positively charged and less massive than beta particles. b. alpha particles are negatively charged and. less massive than beta particles. c. alpha particles are positively charged and more massive than beta particles. d.
Comments are closed.