Solved Dilution Practice Problem 5 You Are Given The Chegg
Solved Dilution Practice Problem 5 You Are Given The Chegg Your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. see answer. Problem #5: a 40.0 ml volume of 1.80 m fe (no 3) 3 is mixed with 21.5 ml of 0.808m fe (no 3) 3 solution. calculate the molar concentration of the final solution.
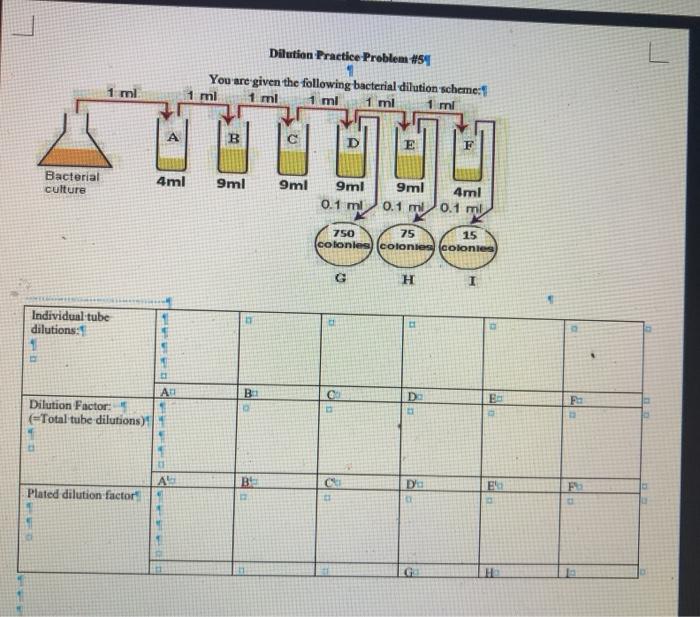
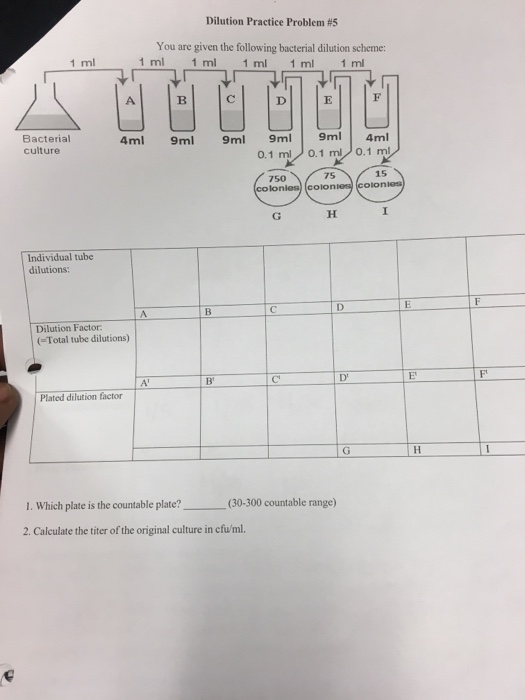
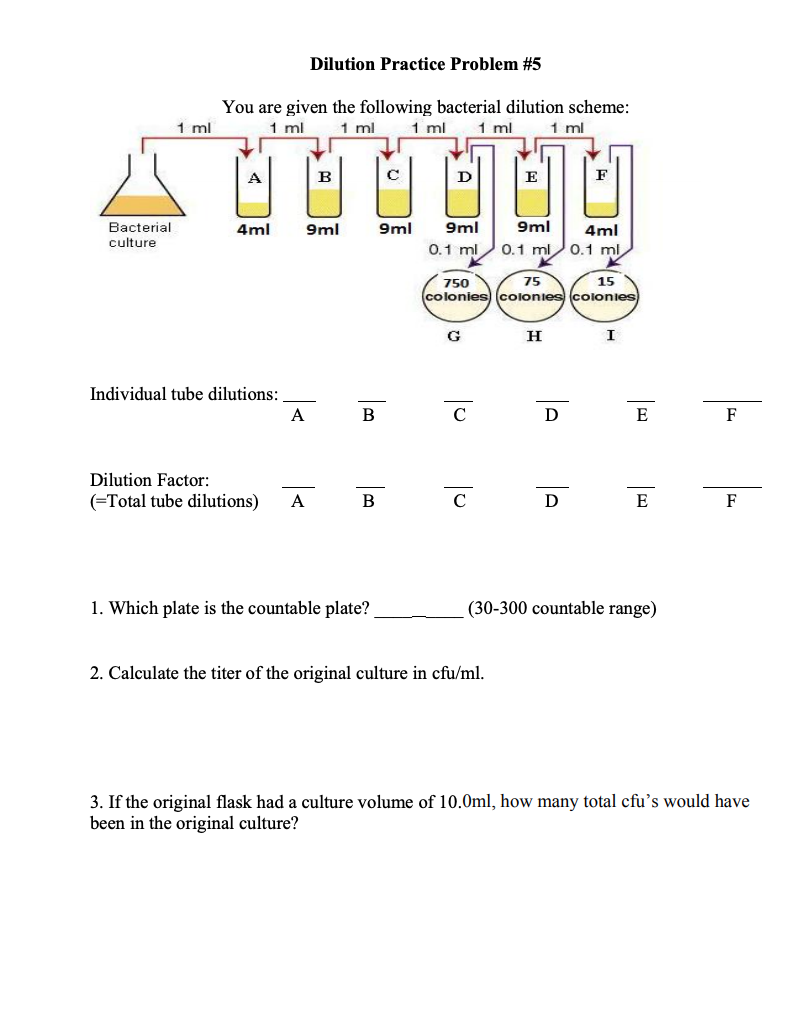
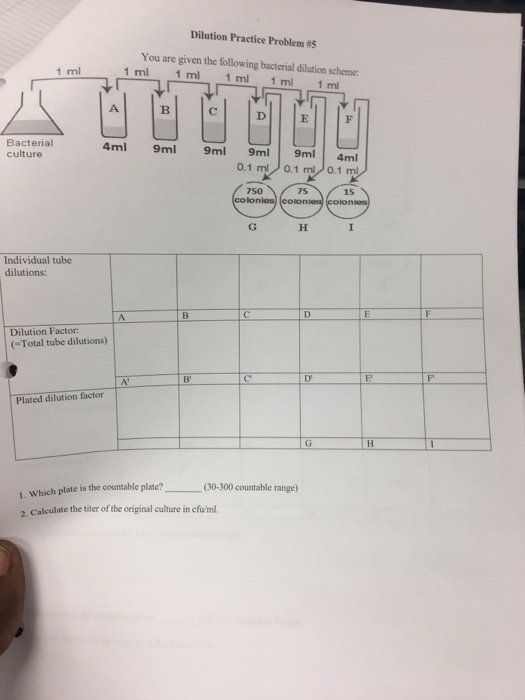
Solved Dilution Practice Problem 5 You Are Given The Chegg Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. you dilute the solution to 600 ml. what is the percent strength of the final solution?, a 20% solution has been diluted to 400 ml and is now a 5% solution. To solve this dilution problem: 1. the countable plate is the one that falls within the range of 30 300 colonies. from the given data, plate c has 15 colonies, plate d has 75 colonies, and plate f has 750 colonies. therefore, plate d is the countable plate since it has 75 colonies, which falls within the countable range. 2. Step 1 prepare a table of the concentrations and volumes of the solutions. step 2 rearrange the dilution expression to solve for the unknown quantity. step 3 substitute the known quantities into the dilution expression and calculate. Practice problems: dilution m1v1=m2v2 1. there’s a bottle of 0.750 m nacl on a shelf. how much of it do you need to prepare 50 ml of a 0.10 m nacl solution? 2. 10.0 ml of 1.00 m hcl is diluted to 100 ml. what is the final concentration? 3. the concentration of a diluted solution with a volume of 150.0 ml is 0.0955 m. it was.
Solved Dilution Practice Problem 5 You Are Given The Chegg Step 1 prepare a table of the concentrations and volumes of the solutions. step 2 rearrange the dilution expression to solve for the unknown quantity. step 3 substitute the known quantities into the dilution expression and calculate. Practice problems: dilution m1v1=m2v2 1. there’s a bottle of 0.750 m nacl on a shelf. how much of it do you need to prepare 50 ml of a 0.10 m nacl solution? 2. 10.0 ml of 1.00 m hcl is diluted to 100 ml. what is the final concentration? 3. the concentration of a diluted solution with a volume of 150.0 ml is 0.0955 m. it was. Problem 6.1.1.1 6.1.1. 1 explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. answer the number of moles always stays the same in a dilution. the concentration and the volumes change in a dilution. Dilution calculations are easy! we just need to know about the concept of concentration, and then the equation m1v1 = m2v2. try some examples!. How would you prepare the 1: 5 dilution from a sediment sample? and the 1:8 dilution? if we plate 0.5 ml on a culture medium and, after incubation, 120 colonies are present in the plate, which is the microbial density of the sample expressed as colony forming units per 100 g of sediment?. Study with quizlet and memorize flashcards containing terms like you have 100 ml of a 2 m hydrochloric acid (hcl) solution. if you dilute this solution to a final volume of 500 ml, what will be the new molarity of the diluted solution?, you have a stock solution of 10 m sodium chloride (nacl).
Solved Dilution Practice Problem 5 You Are Given The Chegg Problem 6.1.1.1 6.1.1. 1 explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. answer the number of moles always stays the same in a dilution. the concentration and the volumes change in a dilution. Dilution calculations are easy! we just need to know about the concept of concentration, and then the equation m1v1 = m2v2. try some examples!. How would you prepare the 1: 5 dilution from a sediment sample? and the 1:8 dilution? if we plate 0.5 ml on a culture medium and, after incubation, 120 colonies are present in the plate, which is the microbial density of the sample expressed as colony forming units per 100 g of sediment?. Study with quizlet and memorize flashcards containing terms like you have 100 ml of a 2 m hydrochloric acid (hcl) solution. if you dilute this solution to a final volume of 500 ml, what will be the new molarity of the diluted solution?, you have a stock solution of 10 m sodium chloride (nacl).
Comments are closed.