Solved Calculating Percent Yield When You Perform A Chegg
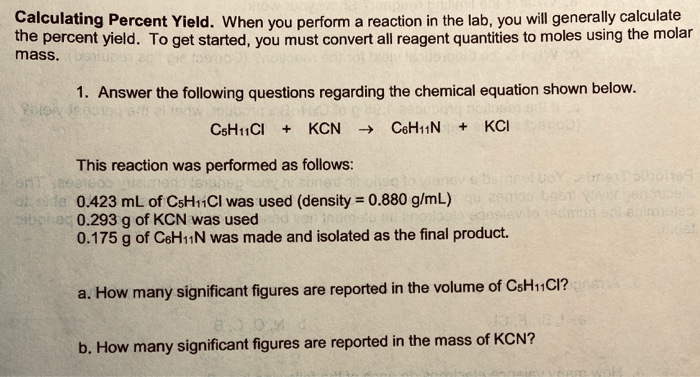
Solved Calculating Percent Yield When You Perform A Chegg When you perform a reaction in the lab, you will generally calculate the percent yield. to get started, you must convert all reagent quantities to moles using the molar mass. 1. answer the following questions regarding the chemical equation shown below. blivit c6hcl kcn c6h11n kci this reaction was performed as follows: dista. Follow these steps to determine the percent yield or, in general, working on stoichiometry problems: 1) determine the moles of reactants. 2) determine the limiting reagent. 3) determine the theoretical yield. 4) determine the reaction percent yield.

Solved Sample Problem 7 14 Calculating Percent Yield On A Chegg Learn how to calculate percent yield in chemistry with this easy, step by step tutorial! we’ll explain the percent yield formula, solve real examples, and walk you through practice problems with. In a laboratory experiment, the reaction of 3.0 mol of h 2 with 2.0 mol of i 2 produced 1.0 mol of hi. determine the theoretical yield in grams and the percent yield for this reaction. Here's a solved example to illustrate how to calculate percentage yield: example 1: what is the percentage yield when conducting a chemical reaction with an expected yield of 50 grams of a certain compound based on stoichiometry, but the actual yield obtained during the reaction is only 42 grams?. Use the balanced equation to find out how many liters of sulfur dioxide are actually produced at stp if 1.5 x 1027 molecules of zinc sulfide are reacted with excess oxygen and the percent yield is 75%.

Solved Percent Yield If You Were To Perform This Reaction In Chegg Here's a solved example to illustrate how to calculate percentage yield: example 1: what is the percentage yield when conducting a chemical reaction with an expected yield of 50 grams of a certain compound based on stoichiometry, but the actual yield obtained during the reaction is only 42 grams?. Use the balanced equation to find out how many liters of sulfur dioxide are actually produced at stp if 1.5 x 1027 molecules of zinc sulfide are reacted with excess oxygen and the percent yield is 75%. Enhanced with ai, our expert help has broken down your problem into an easy to learn solution you can count on. here’s the best way to solve it. calculate the theo not the question you’re looking for? post any question and get expert help quickly. If you perform the experiment, you’ll end up with a smaller amount, the actual yield. to express the efficiency of a reaction, you can calculate the percent yield using this formula: %yield = (actual yield theoretical yield) x 100. Study with quizlet and memorize flashcards containing terms like purpose of the lab (what are we doing?), how do you compare the actual yield to the theoretical yield to find the limiting reactant?, safety precautions (4) and more. To compute the percent yield, it is first necessary to determine how much of the product should be formed based on stoichiometry. this is called the theoretical yield, the maximum amount of product that could be formed from the given amounts of reactants.
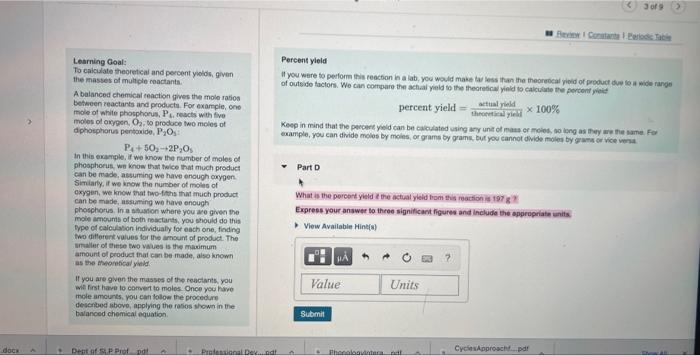
Solved Percent Yield If You Were To Perform This Reaction In Chegg Enhanced with ai, our expert help has broken down your problem into an easy to learn solution you can count on. here’s the best way to solve it. calculate the theo not the question you’re looking for? post any question and get expert help quickly. If you perform the experiment, you’ll end up with a smaller amount, the actual yield. to express the efficiency of a reaction, you can calculate the percent yield using this formula: %yield = (actual yield theoretical yield) x 100. Study with quizlet and memorize flashcards containing terms like purpose of the lab (what are we doing?), how do you compare the actual yield to the theoretical yield to find the limiting reactant?, safety precautions (4) and more. To compute the percent yield, it is first necessary to determine how much of the product should be formed based on stoichiometry. this is called the theoretical yield, the maximum amount of product that could be formed from the given amounts of reactants.
Solved Please I Need Help In Calculating The Percent Yield Chegg Study with quizlet and memorize flashcards containing terms like purpose of the lab (what are we doing?), how do you compare the actual yield to the theoretical yield to find the limiting reactant?, safety precautions (4) and more. To compute the percent yield, it is first necessary to determine how much of the product should be formed based on stoichiometry. this is called the theoretical yield, the maximum amount of product that could be formed from the given amounts of reactants.
Comments are closed.