Solved 2 Explain The Possible Effects On The Results For Chegg
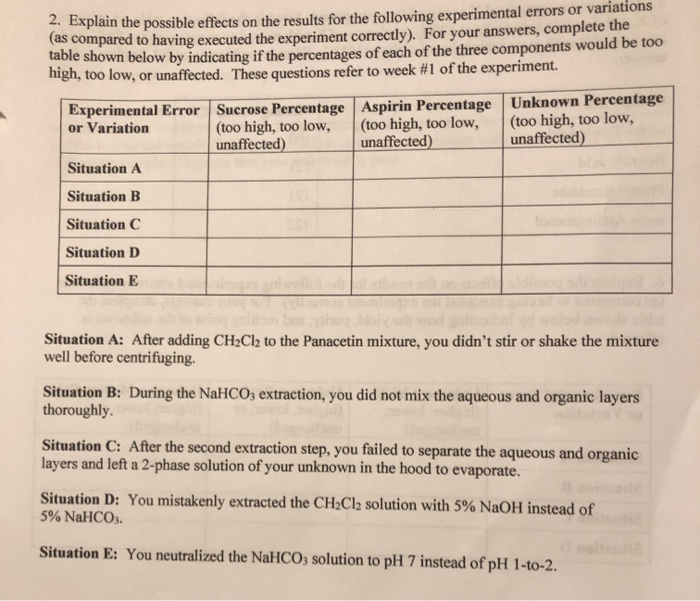
Solved 2 Explain The Possible Effects On The Results For Chegg Explain the possible effects on the results for the following experimental errors or variations (as compared to having executed the experiment correctly). for your answers, complete the table shown below by indicating if the percentages of each of the three components would be too high, too low, or unaffected. Study with quizlet and memorize flashcards containing terms like describe and explain the possible effect on your results of the following experimental errors or variations.

Solved Describe And Explain The Possible Effects On Your Chegg The possible effects on the results due to the above mentioned experimental errors are as follows. a) if you allowed your chromatogram to develop too long and couldn't find the solvent front, this could cause your samples to run together. this would make it difficult to identify separate components, reducing the reliability of your results. Depending on the specific reaction, this could lead to different products being formed or a change in the yield of the desired product. the results would again be less accurate and potentially skewed. in conclusion, both experimental errors or variations could lead to inaccurate and skewed results. Describe and explain the possible effect on your results of the following experimental errors or variations. a. you forgot to add the phosphoric acid. b. you used a simple distillation apparatus instead of a fractional distillation setup with a vigreux column. c. Describe and explain the possible effect on your results if you used enough water to recrystallize phenacetin, but your unknown was acetanilide. acetanilide is far more soluble in boiling water than phenacetin. therefore, less. water would be necessary to dissolve acetanilide than phenacetin.

Solved 6 Explain The Possible Effects On The Results For Chegg Describe and explain the possible effect on your results of the following experimental errors or variations. a. you forgot to add the phosphoric acid. b. you used a simple distillation apparatus instead of a fractional distillation setup with a vigreux column. c. Describe and explain the possible effect on your results if you used enough water to recrystallize phenacetin, but your unknown was acetanilide. acetanilide is far more soluble in boiling water than phenacetin. therefore, less. water would be necessary to dissolve acetanilide than phenacetin. There are several factors that could support different results in your findings. here are some of them: data quality: the quality of your data can significantly impact your results. if your data is inaccurate, incomplete, or biased, your regression analysis may not accurately reflect the true relationship between listing prices and square footage. The reaction of the pure trans isomer with phosphoric acid may produce different products than the reaction of a mixture of isomers. this can affect the purity and yield of the final product. conclusion: experimental errors or variations can have a significant effect on the results of an experiment. Describe and explain the possible effect on your results of the following experimental errors or variations. (a) the reaction test tube contained water. (b) you heated the oil with methanolic sodium hydroxide but forgot to add the boron trifluoride methanol solution. your solution’s ready to go!. Find step by step chemistry solutions and the answer to the textbook question describe and explain the possible effect on your results of the following experimental errors or variations. in each case specify the component (s) whose percentage (s) would be too high or too low.

Explain Your Results Chegg There are several factors that could support different results in your findings. here are some of them: data quality: the quality of your data can significantly impact your results. if your data is inaccurate, incomplete, or biased, your regression analysis may not accurately reflect the true relationship between listing prices and square footage. The reaction of the pure trans isomer with phosphoric acid may produce different products than the reaction of a mixture of isomers. this can affect the purity and yield of the final product. conclusion: experimental errors or variations can have a significant effect on the results of an experiment. Describe and explain the possible effect on your results of the following experimental errors or variations. (a) the reaction test tube contained water. (b) you heated the oil with methanolic sodium hydroxide but forgot to add the boron trifluoride methanol solution. your solution’s ready to go!. Find step by step chemistry solutions and the answer to the textbook question describe and explain the possible effect on your results of the following experimental errors or variations. in each case specify the component (s) whose percentage (s) would be too high or too low.

Solved Describe And Explain The Possible Effect On Your Chegg Describe and explain the possible effect on your results of the following experimental errors or variations. (a) the reaction test tube contained water. (b) you heated the oil with methanolic sodium hydroxide but forgot to add the boron trifluoride methanol solution. your solution’s ready to go!. Find step by step chemistry solutions and the answer to the textbook question describe and explain the possible effect on your results of the following experimental errors or variations. in each case specify the component (s) whose percentage (s) would be too high or too low.
Comments are closed.