Solved 1 The Compression And Expansion Ratio Of An Oil Chegg
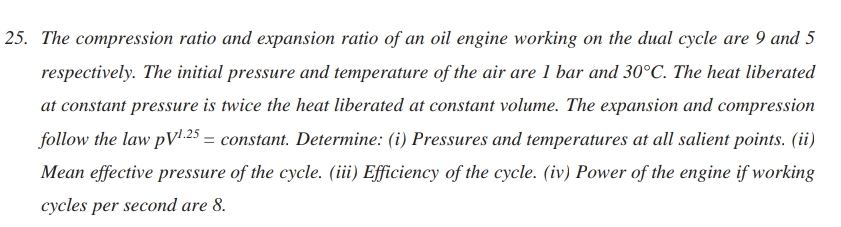
Solved The Compression Ratio And Expansion Ratio Of An Oil Chegg 1) the compression and expansion ratio of an oil engine working on dual cycle are 9 and 5 respectively. the initial pressure and temperature of air are 1bar and 30°c. the heat added at constant pressure is twice that of heat added at constant volume. the compression and expansion follow the law pv1.4=c. The compression ratio and expansion ratio of an oil engine working on the dual cycle are 9 and 5 respectively. the initial pressure and temperature of the air are 1 bar and 30°c. the heat liberated at constant pressure is twice the heat liberated at constant volume. the expansion and compression follow the law p v^ {1.25}= pv 1.25 = constant.
Solved Determine The Ratio Of The Compression Work To The Chegg The compression ratio and expansion ratio of an oil engine working on the dual cycle are 9 and 5 respectively. the initial pressure and temperature of the air are 1 bar and 30°c. An oil engine takes in air at 1.01 bar, 20 oc and the maximum cycle pressure is 69 bar. the compressor ratio is 18:1. calculate the air standard thermal efficiency based on the dual combustion cycle. assume that the heat added at constant volume is equal to the heat added at constant pressure. answer: n = 68.2% solution (36 kbytes) problem 6: 4. The pressure and temperature of air at the beginning of compression in an otto cycle is 100 kpa and 300 k, respectively. the heat added per kg of air is 1436 kj. Problem 3 1 calculates temperatures, pressures, specific work, heat added, net work, and indicated thermal efficiency for an otto cycle engine given initial cylinder conditions and compression ratio.

Solved Question 1 0 5 Pts The Compression Ratio Is The Ratio Chegg The pressure and temperature of air at the beginning of compression in an otto cycle is 100 kpa and 300 k, respectively. the heat added per kg of air is 1436 kj. Problem 3 1 calculates temperatures, pressures, specific work, heat added, net work, and indicated thermal efficiency for an otto cycle engine given initial cylinder conditions and compression ratio. The compression ratio and expansion ratio of an oil engine working on the dual cycle are 9 and 5 respectively. the initial pressure and temperature of the air are 1 bar and 30°c. the heat liberated at constant pressure is twice the heat liberated at constant volume. the expansion and compression follow the law p v^ {1.25} v 1.25 = constant. The expansion and compression follow the law p v1.25= constant. determine: (i) the compression ratio and expansion ratio of an oil engine working on the dual cycle are 9 and 5 respectively. the initial pressure and temperature of the air are 1 bar and 3 0 ° c. Determine the % correction to the ideal gas pressure at a temperature 200 k and atmospheric pressure due to the first term in the virial expansion (i.e. the term due to. To quantify just how compressible substances are, it is necessary to define the property. the isothermal compressibility is defined by the fractional differential change in volume due to a change in pressure. κt ≡ − 1 v(∂v ∂p)t (4.3.1) (4.3.1) κ t ≡ 1 v (∂ v ∂ p) t.

Solved Que 2 Attempt Any One 05 An Oil Engine Working On Chegg The compression ratio and expansion ratio of an oil engine working on the dual cycle are 9 and 5 respectively. the initial pressure and temperature of the air are 1 bar and 30°c. the heat liberated at constant pressure is twice the heat liberated at constant volume. the expansion and compression follow the law p v^ {1.25} v 1.25 = constant. The expansion and compression follow the law p v1.25= constant. determine: (i) the compression ratio and expansion ratio of an oil engine working on the dual cycle are 9 and 5 respectively. the initial pressure and temperature of the air are 1 bar and 3 0 ° c. Determine the % correction to the ideal gas pressure at a temperature 200 k and atmospheric pressure due to the first term in the virial expansion (i.e. the term due to. To quantify just how compressible substances are, it is necessary to define the property. the isothermal compressibility is defined by the fractional differential change in volume due to a change in pressure. κt ≡ − 1 v(∂v ∂p)t (4.3.1) (4.3.1) κ t ≡ 1 v (∂ v ∂ p) t.

Solved 1 Figure 1 Illustrates The Compression Expansion Chegg Determine the % correction to the ideal gas pressure at a temperature 200 k and atmospheric pressure due to the first term in the virial expansion (i.e. the term due to. To quantify just how compressible substances are, it is necessary to define the property. the isothermal compressibility is defined by the fractional differential change in volume due to a change in pressure. κt ≡ − 1 v(∂v ∂p)t (4.3.1) (4.3.1) κ t ≡ 1 v (∂ v ∂ p) t.
Comments are closed.