Nh 3n And No 3 N Contents In Heap 2 Of Cycle 6 Download Scientific

Nh 3n And No 3 N Contents In Heap 2 Of Cycle 6 Download Scientific These so called diazotrophs are capable of reducing n 2 to ammonia (nh 3 ) via the nitrogenase enzyme and supposedly comprise a significant source of fixed nitrogen to coral holobiont. 5 the nitrogen cycle n2 is reduced to organic n organisms anaerobic bacteria in the roots of leguminous plants algae or cyanobacteria in the ocean (mainly trichodesmium) are consumed heterotrophs which release ammonia nitrogen fixation transforms n2 fixed nitrogen 1.

Solved Nh 3n O E Nole 3h2 G N H 0 0 G 4n2 8 3h2o Chegg Under normal conditions, electron loss to for n , n2 or n3 or electron gain to form n , n2 , or n3 should not be expected. instead, n will normally fill its 2p orbital by sharing electrons with other elements to which it is chemically (covalent) bound. Conversion of atmospheric n to ammonia requires the enzyme nitrogenase. the conversion of organic nitrogen to nh 3 is called ammonification. in the presence of water, nh 3 becomes ionized and forms ammonium (nh 4 ). Ammonia can be used directly as fertilizer, but most of it is further processed to urea and ammonium nitrate (nh 4 no 3). the third process is biological fixation by certain free living or symbiotic bacteria, which incorporate nitrogen into their macromolecules. This complex cycle involves bacteria, plants, and animals. all organisms can convert ammonia (nh3) to organic nitrogen compounds that are compounds containing c–n bonds. however, only a few microorganisms can synthesize ammonia from nitrogen gas (n2).
Solved N Nh C6hs N C6hs N Nh2 Ii Iii O N N C6hs Iz Nh2 Iv V Which Ammonia can be used directly as fertilizer, but most of it is further processed to urea and ammonium nitrate (nh 4 no 3). the third process is biological fixation by certain free living or symbiotic bacteria, which incorporate nitrogen into their macromolecules. This complex cycle involves bacteria, plants, and animals. all organisms can convert ammonia (nh3) to organic nitrogen compounds that are compounds containing c–n bonds. however, only a few microorganisms can synthesize ammonia from nitrogen gas (n2). The entire process of the nitrogen cycle, one of the important biogeochemical cycle takes place in five stages: 1) nitrogen fixation by bacteria – converting inert atmospheric nitrogen (n 2)into biologically available forms such as ammonia (nh 3), nitrates, or nitrites. Nitrogen must first be transformed from n 2 gas into a more chemically usable form, such as ammonium (nh 4 ), nitrate (no 3–), or organic nitrogen (for example, urea (nh 2) 2 co). The nitrogen cycle has 5 basic steps 1) nitrogen fixation: n2 => nh3 root nodules on a legume. Nitrogen in rocks is generally in the form of nh 4 in silicates (clays), although a few no 3 − bearing minerals are known. in igneous rocks, n content is usually <0.1 wt%. nitrogen in sedimentary rocks can be much higher (up to ~2 wt%).
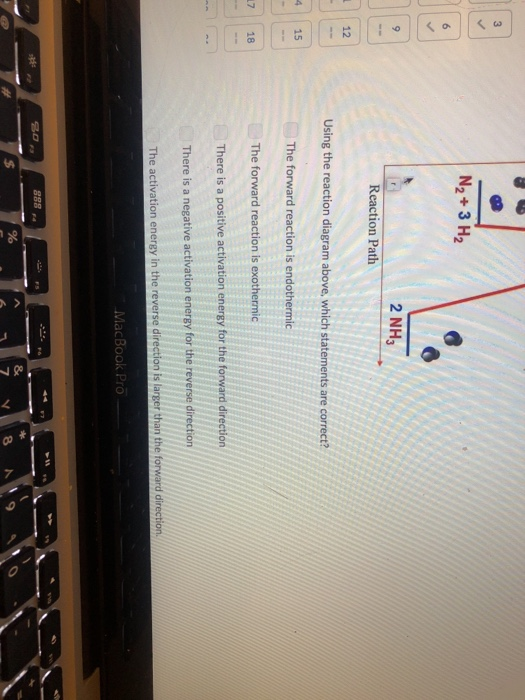
Solved N2 3 H2 6 2 Nh3 9 Reaction Path 12 Using The Reaction Chegg The entire process of the nitrogen cycle, one of the important biogeochemical cycle takes place in five stages: 1) nitrogen fixation by bacteria – converting inert atmospheric nitrogen (n 2)into biologically available forms such as ammonia (nh 3), nitrates, or nitrites. Nitrogen must first be transformed from n 2 gas into a more chemically usable form, such as ammonium (nh 4 ), nitrate (no 3–), or organic nitrogen (for example, urea (nh 2) 2 co). The nitrogen cycle has 5 basic steps 1) nitrogen fixation: n2 => nh3 root nodules on a legume. Nitrogen in rocks is generally in the form of nh 4 in silicates (clays), although a few no 3 − bearing minerals are known. in igneous rocks, n content is usually <0.1 wt%. nitrogen in sedimentary rocks can be much higher (up to ~2 wt%).
Comments are closed.