How To Do Solution Stoichiometry Using Molarity As A Conversion F

Stoichiometry Conversion Practice Pdf Practice problem: a 34.53 ml sample of h2so4 reacts with 27.86 ml of 0.08964 m naoh solution. calculate the molarity of the h2so4 solution. Because these reactions occur in aqueous solution, we can use the concept of molarity to directly calculate the number of moles of reactants or products that will be formed, and therefore their amounts (i.e. volume of solutions or mass of precipitates).
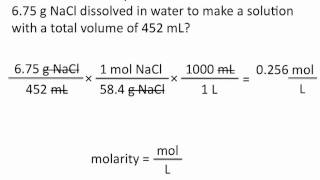
How To Do Solution Stoichiometry Using Molarity As A Conversion F Molarity can be used as a conversion factor in combination with reaction stoichiometry. 1. magnesium reacts with hydrochloric acid to produce hydrogen gas and magnesium chloride. how many liters of a 0.750 m hcl solution will react with 12.25 g of mg? mg(s) 2 hcl(aq) → h2(g) mgcl2(aq) mg (s) 2 hcl (a q) → h 2 (g) mg cl 2 (a q) 2. Chemical quantities & aqueous reactions molarity. struggling with general chemistry? join thousands of students who trust us to help them ace their exams! watch the first video. Whenever the solution concentration is given in molarity, m, you must change to the equivalent units, mol l or mol 1000 ml, to use as a conversion factor. example: what mass of solute is contained in 15.0 ml of a 0.760 m kcl solution? m solution? 1 l 250.0 ml x 1000 ml. 1000 ml. Use the mole ratios from the balanced chemical equation to convert from moles of limiting reactant to moles of product and then use the gram mole conversion factor from the periodic table to determine the grams of product formed.

Conversions Solution Stoichiometry Whenever the solution concentration is given in molarity, m, you must change to the equivalent units, mol l or mol 1000 ml, to use as a conversion factor. example: what mass of solute is contained in 15.0 ml of a 0.760 m kcl solution? m solution? 1 l 250.0 ml x 1000 ml. 1000 ml. Use the mole ratios from the balanced chemical equation to convert from moles of limiting reactant to moles of product and then use the gram mole conversion factor from the periodic table to determine the grams of product formed. Given the molarity of a substance, write a conversion factor that relates the moles of substance to liters of solution and a conversion factor that relates the moles of solute to milliliters of solution. Our goal is to calculate the concentration in moles per liter or, equivalently, to determine the molarity of the solution. we begin by writing down our initial conditions. we have 2.00 grams of glucose per 100 ml of solution. first, we convert from grams of glucose to moles of glucose. to do this, we need the molecular weight of glucose. In this example, we convert from ml of solution to grams of solute, using molarity as a conversion factor in dimensional analysis. Chemists express the concentration of a solution using molarity (m), the number of moles of solute per litre of solution. by combining this with balanced chemical equations, we can perform solution stoichiometry calculations to determine how much of each substance reacts or forms in an aqueous process. in this section, you'll learn how to calculate molarity, perform dilutions, and use volume.

Conversions Solution Stoichiometry Given the molarity of a substance, write a conversion factor that relates the moles of substance to liters of solution and a conversion factor that relates the moles of solute to milliliters of solution. Our goal is to calculate the concentration in moles per liter or, equivalently, to determine the molarity of the solution. we begin by writing down our initial conditions. we have 2.00 grams of glucose per 100 ml of solution. first, we convert from grams of glucose to moles of glucose. to do this, we need the molecular weight of glucose. In this example, we convert from ml of solution to grams of solute, using molarity as a conversion factor in dimensional analysis. Chemists express the concentration of a solution using molarity (m), the number of moles of solute per litre of solution. by combining this with balanced chemical equations, we can perform solution stoichiometry calculations to determine how much of each substance reacts or forms in an aqueous process. in this section, you'll learn how to calculate molarity, perform dilutions, and use volume.
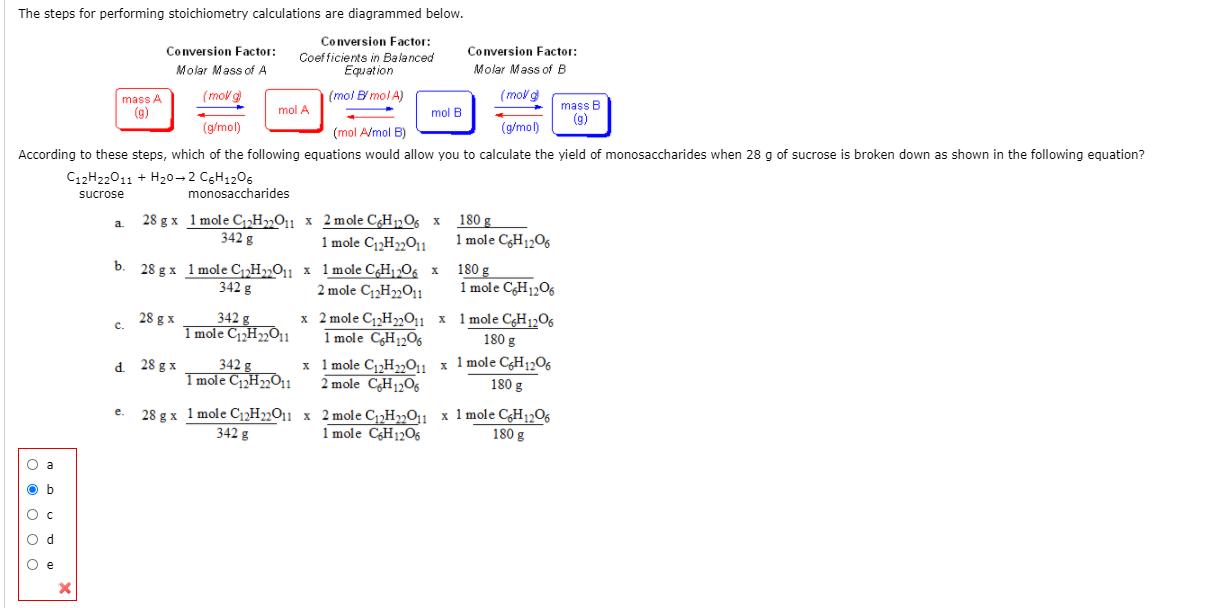
Solved The Steps For Performing Stoichiometry Calculations Chegg In this example, we convert from ml of solution to grams of solute, using molarity as a conversion factor in dimensional analysis. Chemists express the concentration of a solution using molarity (m), the number of moles of solute per litre of solution. by combining this with balanced chemical equations, we can perform solution stoichiometry calculations to determine how much of each substance reacts or forms in an aqueous process. in this section, you'll learn how to calculate molarity, perform dilutions, and use volume.
Comments are closed.