Gcse Chemistry Revision Calculating Percentage Yield 1 Triple
Percentage Yield Triple Gcse Chemistry Worksheets With Answers In this video, which is the first of two, we look at how to calculate the percentage yield for a reaction. we learn why percentage yield is rarely 100% and then i explain why students. Learn how to calculate percentage yield in gcse chemistry. understand the difference between actual and theoretical yield, and explore factors affecting yield.

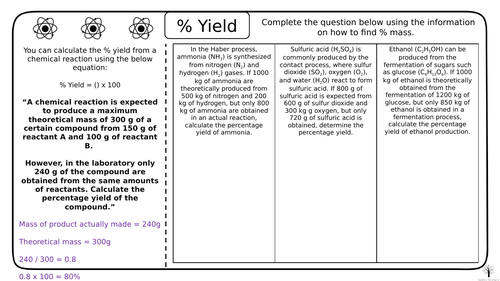
Chemistry Calculating Percentage Yield Teaching Resources The percentage yield shows how much product is obtained compared to the maximum possible mass. the atom economy of a reaction gives the percentage of atoms in reactants that form a desired. Percentage yield is calculated using the following formula: percentage yield = (actual yield ÷ theoretical yield) x 100. the actual yield refers to the amount of product that is actually produced in a reaction, while the theoretical yield refers to the maximum amount of product that could be produced based on the amount of reactants used. It’s calculated using the formula: (actual yield theoretical yield) x 100%. the actual yield is the amount of product you actually get from a reaction. this can be less than the theoretical yield due to various possible losses during the experimental process. Calculating percentage yield 1 back to gcse chemistry gcse chemistry revision "calculating percentage yield 1" (triple) watch on 0:00 4:12.

Chemistry Calculating Percentage Yield By Science Lessons 4 You Tpt It’s calculated using the formula: (actual yield theoretical yield) x 100%. the actual yield is the amount of product you actually get from a reaction. this can be less than the theoretical yield due to various possible losses during the experimental process. Calculating percentage yield 1 back to gcse chemistry gcse chemistry revision "calculating percentage yield 1" (triple) watch on 0:00 4:12. The formula for calculating percentage yield is: in an experiment, aluminium oxide reacts with sulfuric acid to form aluminium sulfate and water. the balanced equation for the reaction is: aluminium oxide sulphuric acid → aluminium sulfate water. al 2 o 3 3h 2 so 4 → al 2 (so 4) 3 3h 2 o. This resource contains 5 worksheets for ‘percentage yield’ in ‘quantiative chemistry’ that can be used in class or as homework to enable your students to practice what they have learnt in the classroom. worksheets include: using the % yield equation rearranging the % yield equation calculating % yield from balanced symbol equations (ht). Exam pro questions for unit 3 these questions are mixed knowledge, you need to focus on calculations so skip down until you find calculate questions. click on the link , answer the questions and use the mark scheme tab to mark them. you do not have to print them just use paper. Percentage purity of a substance can be calculated by dividing the mass of the pure chemical by the total mass of the sample, and then multiplying this number by 100. learn about chemical.
Comments are closed.