Developed An End To End Language Learning Mobile Application With

Developed An End To End Language Learning Mobile Application With An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in an ionic compound. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom.
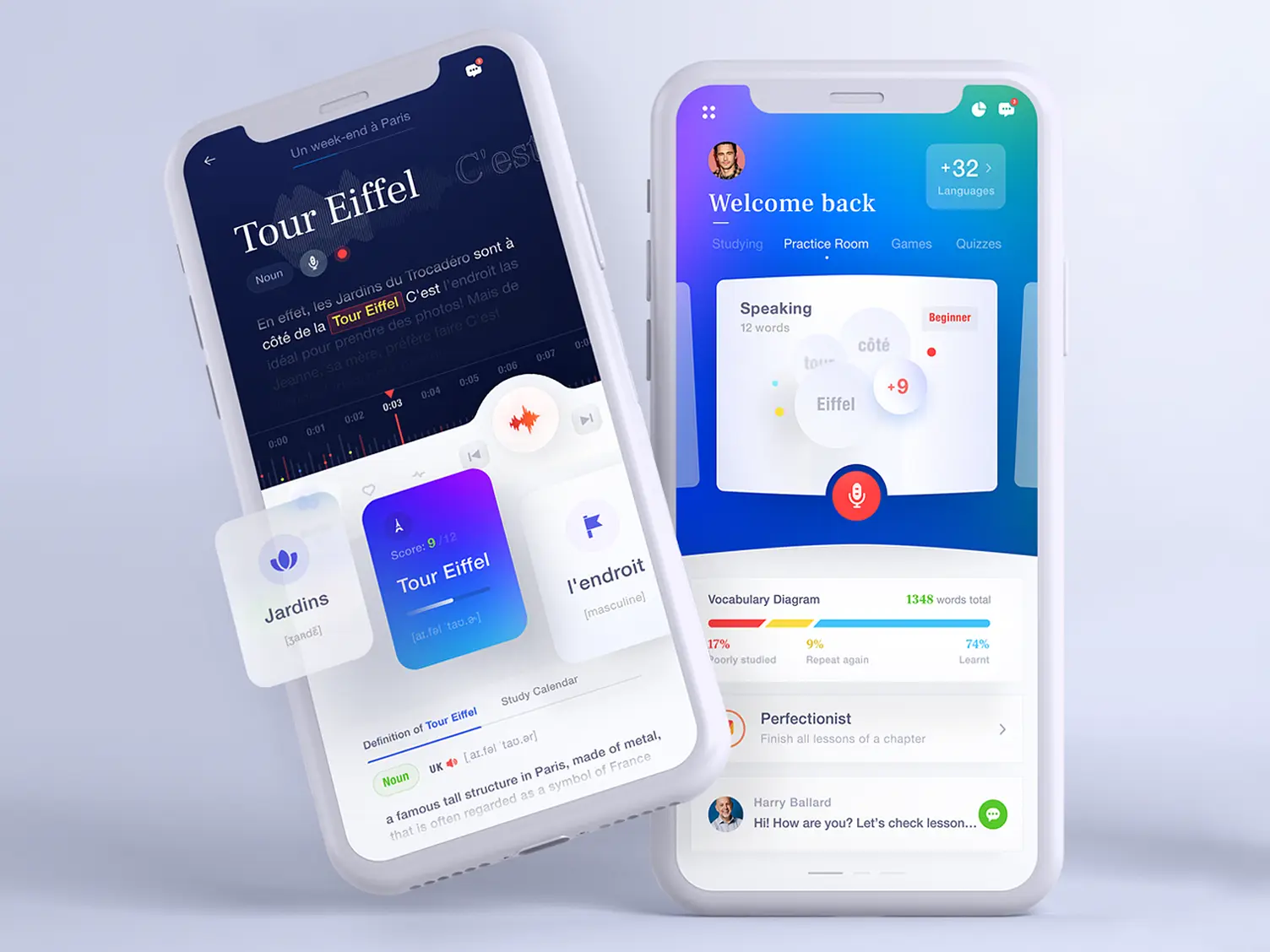
Language Learning App Mobile Application Design Concept Ramotion Agency An ionic bond or electrovalent bond is an electrostatic attraction where one atom donates an electron to another atom. the transfer results in the atom that loses an electron become a positively charged ion or cation, while the atom gaining the electron becomes a negatively charged ion or anion. An ionic bond is the bond formed by the complete transfer of valence electron to attain stability. this type of bonding leads to the formation of two oppositely charged ions – positive ions known as cations and negative ions known as anions. Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, [1] and is the primary interaction occurring in ionic compounds. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties.

Mobile Application For Learning Languages On Behance Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, [1] and is the primary interaction occurring in ionic compounds. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. Ionic bonding is the attraction between positively and negatively charged ions. these oppositely charged ions attract each other to form ionic networks (or lattices). electrostatics explains why this happens: opposite charges attract and like charges repel. The ionic bond is a type of chemical interaction or linkage as a result of electrostatic attraction between oppositely charged ions or atoms having different electronegativities. Find everything you need to teach ionic bonding to learners aged 14–16 years in our resource package, including worksheets, lesson plans, experiments, language support and enrichment. Ionic bonds are formed when metal atoms transfer electrons to non metal atoms. this is done so that each atom forms an ion with a full outer shell of electrons. example 1: the formation of an ionic bond between chlorine and sodium. example 2: the formation of the ionic bond between oxygen and magnesium. glossary of terms:.

Mobile Application For Learning Languages On Behance Ionic bonding is the attraction between positively and negatively charged ions. these oppositely charged ions attract each other to form ionic networks (or lattices). electrostatics explains why this happens: opposite charges attract and like charges repel. The ionic bond is a type of chemical interaction or linkage as a result of electrostatic attraction between oppositely charged ions or atoms having different electronegativities. Find everything you need to teach ionic bonding to learners aged 14–16 years in our resource package, including worksheets, lesson plans, experiments, language support and enrichment. Ionic bonds are formed when metal atoms transfer electrons to non metal atoms. this is done so that each atom forms an ion with a full outer shell of electrons. example 1: the formation of an ionic bond between chlorine and sodium. example 2: the formation of the ionic bond between oxygen and magnesium. glossary of terms:.
Comments are closed.