Concept Of Valency Introduction Atoms And Molecules Infinity Learn

Valency And Formulae Handout Pdf Valence Chemistry Ion Valency is the number of atoms of a particular element that is combined with one atom of another element to form a molecule. valency is also known as molecular weight. Valence is a concept used to quantify covalent bonding between atoms in molecules rather than ionic bonding. the driving force for a quantitative fixation in a convenient valence index was chemical reactivity.
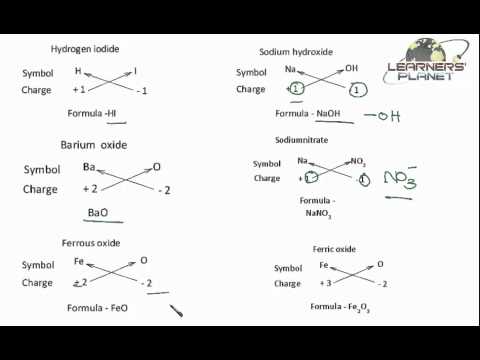
Valency And Writing Chemical Formula Atoms And Molecules Video Here, we dive into three core concepts that will unlock the mysteries of molecular behavior: mole, valency, and equivalence. these concepts are central to almost every aspect of chemistry and will help you understand how substances interact at the atomic level. It explains methods to determine the valency of an element using electron configurations, discusses normal and excited states of atoms, and outlines how to define chemical formulas based on valency. To introduce the basic principles of covalent bonding, different types of molecular representations, bond polarity and its role in electronic density distributions, and physical properties of molecules. they are those found in the highest energy level of the atom, or outer shell. Valency refers to to the number of electrons in the outermost (valence) electron orbital of an atom or ion. it’s used to determine (in general) the reactivity of an atom in a given situation. the mole is a theoretical concept used to describe the amount of a given substance you are measuring.

What Is Valency Difference Between Valency Oxidation Number To introduce the basic principles of covalent bonding, different types of molecular representations, bond polarity and its role in electronic density distributions, and physical properties of molecules. they are those found in the highest energy level of the atom, or outer shell. Valency refers to to the number of electrons in the outermost (valence) electron orbital of an atom or ion. it’s used to determine (in general) the reactivity of an atom in a given situation. the mole is a theoretical concept used to describe the amount of a given substance you are measuring. 🫀 valency governs how atoms interact by determining electron sharing or exchange. understanding electronic configuration and valency is crucial in explaining chemical reactions. Watch this video which explains how valency plays an important role in the formation of compounds. to learn more about atoms and molecules, enroll in our full course now:. In its simplest form, valence can be defined as the combining capacity of an atom, which is the ability of an atom to form chemical bonds with other atoms. valence electrons, also known as valence shell electrons, are the outermost electrons in an atom’s electron configuration. A valency chart is a diagram that illustrates how many atoms of a particular element are bonded to each atom of another element in a molecule. the valency of an element is the number of bonds it can form.

Concept Of Valency Atoms And Molecules Don T Memorise Youtube 🫀 valency governs how atoms interact by determining electron sharing or exchange. understanding electronic configuration and valency is crucial in explaining chemical reactions. Watch this video which explains how valency plays an important role in the formation of compounds. to learn more about atoms and molecules, enroll in our full course now:. In its simplest form, valence can be defined as the combining capacity of an atom, which is the ability of an atom to form chemical bonds with other atoms. valence electrons, also known as valence shell electrons, are the outermost electrons in an atom’s electron configuration. A valency chart is a diagram that illustrates how many atoms of a particular element are bonded to each atom of another element in a molecule. the valency of an element is the number of bonds it can form.
Comments are closed.