Compression Vs Expansion

Review Of Expansion Compression Of Gases Pdf Gases Thermodynamics Isothermal and isentropic gas compression and expansion processes. the relationship between pressure and density when compressing or expanding a gas depends on the nature of the process. On this page we derive two important equations which relate the pressure and temperature of a gas to the volume which the gas occupies during the compression and power strokes of an internal combustion engine.

Expansion And Compression Ratio Vs Eccentricity Download Scientific This means that, during compression, the irreversible work done by the imposed external force per unit area on the gas is always greater than the reversible work that would have been required to compress the gas, while, during expansion, the irreversible work done against the imposed external force per unit area by the gas is always less than. Expansion cools gases like nitrogen and argon until they liquefy for storage in portable cylinders. lower pressure gases then vaporize as they exit the cylinder and expand. carefully controlling flows prevents damage from expanding gases, cooling, and condensing moisture inside regulators or lines. Compressibility is the property of a gas of being able to decrease in volume under the effect of a force. expansion is the property of a gas of being able to dilate to occupy all the space available to it. the gaseous state is one of three states of matter, the others being the solid and liquid phases. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!) solids and liquids however are not as compressible. however, they are not entirely incompressible! high pressure will lead to a decrease in volume, even if it is only slight.

Expansion Compression On Behance Compressibility is the property of a gas of being able to decrease in volume under the effect of a force. expansion is the property of a gas of being able to dilate to occupy all the space available to it. the gaseous state is one of three states of matter, the others being the solid and liquid phases. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!) solids and liquids however are not as compressible. however, they are not entirely incompressible! high pressure will lead to a decrease in volume, even if it is only slight. The energy required to compress a gas, or the energy obtained from expansion, can be estimated by calculating the ideal work and applying a suitable efficiency value. A discussion on the expansion and compression of ideal gases, also considering the particular cases of isothermal and adiabatic processes references for expansion and compression of ideal gases with worked examples. In most thermodynamic calculations we are dealing with the evaluation of work involved in the expansion or compression of gases. the essential condition for expansion or compression of a system is that there should be difference between external pressure (pext) and internal pressure (pint). The work done by the gas on the piston during irreversible expansion is less than the work done by the piston on the gas during irreversible compression. in either expansion or compression, a rough approximation to the force f on the piston in the irreversible behavior of the gas is given by:.

Expansion Compression Behance The energy required to compress a gas, or the energy obtained from expansion, can be estimated by calculating the ideal work and applying a suitable efficiency value. A discussion on the expansion and compression of ideal gases, also considering the particular cases of isothermal and adiabatic processes references for expansion and compression of ideal gases with worked examples. In most thermodynamic calculations we are dealing with the evaluation of work involved in the expansion or compression of gases. the essential condition for expansion or compression of a system is that there should be difference between external pressure (pext) and internal pressure (pint). The work done by the gas on the piston during irreversible expansion is less than the work done by the piston on the gas during irreversible compression. in either expansion or compression, a rough approximation to the force f on the piston in the irreversible behavior of the gas is given by:.
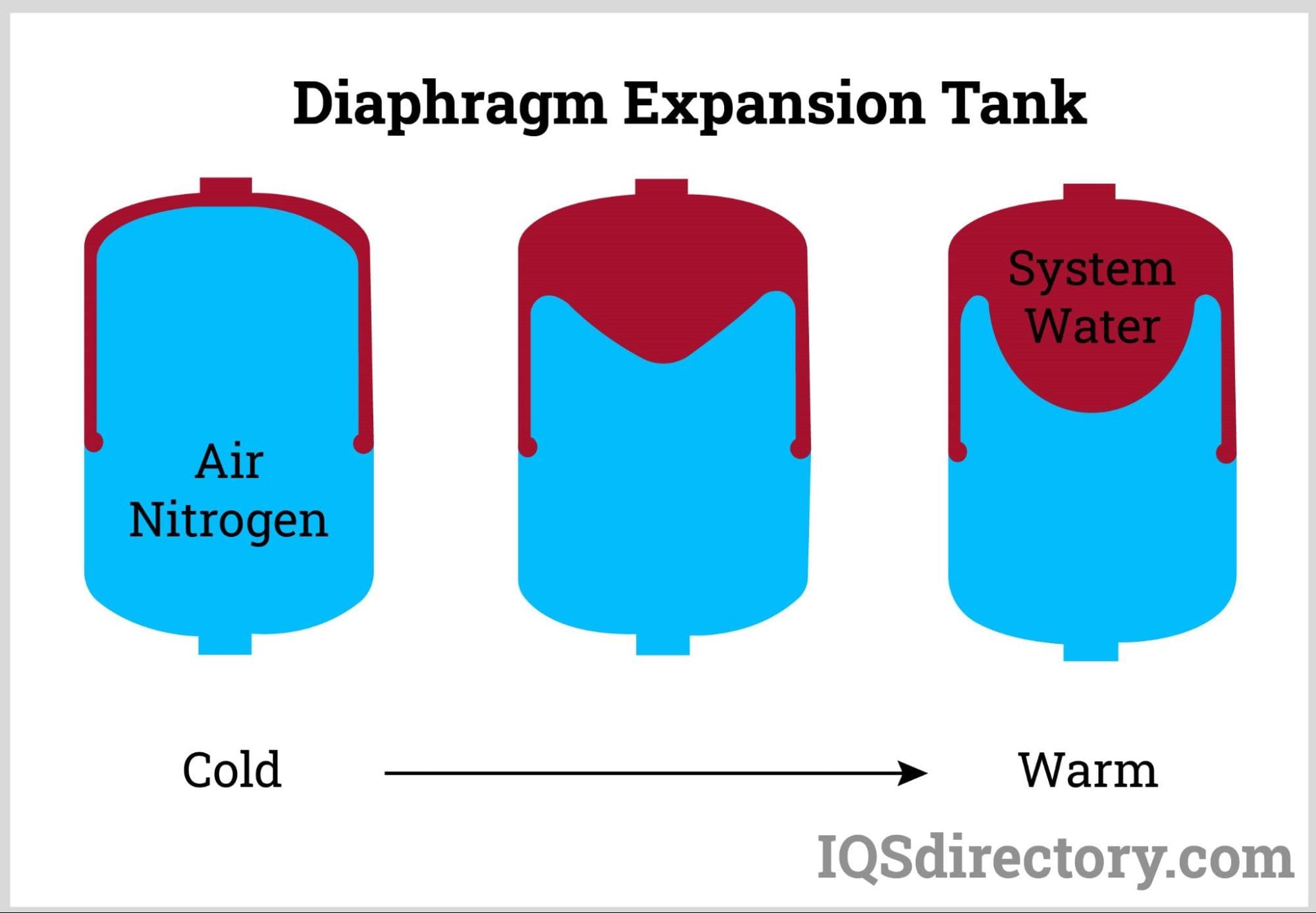
Expansion Tank Vs Compression Tank At Cindy Rahman Blog In most thermodynamic calculations we are dealing with the evaluation of work involved in the expansion or compression of gases. the essential condition for expansion or compression of a system is that there should be difference between external pressure (pext) and internal pressure (pint). The work done by the gas on the piston during irreversible expansion is less than the work done by the piston on the gas during irreversible compression. in either expansion or compression, a rough approximation to the force f on the piston in the irreversible behavior of the gas is given by:.

Compression And Expansion On Behance
Comments are closed.