Calculate The Equilibrium Constant Kc Forthe Formation Of Nh_3in The Following Reactions N_

Solved The Equilibrium Constant Kc For The Following Chegg Calculate the equilibrium constant (k c) for the formation of nh 3 in the following reaction: n a 2 (g) 3 h a 2 (g) ↽ ⇀ 2 nh a 3 (g) at equilibrium, the concentration of nh 3, h 2 and n 2 are 1.2 × 10 2, 3.0 × 10 2 and 1.5 × 10 2 m respectively. n 2 3h 2 ⇌ 2nh 3. k c = nh n h [nh 3] 2 [n 2] [h 2] 3 = (1.2 × 10 2) 2 (1.5 × 10 2) (3 × 10 2) 3. N2(g) 3h2(g)⇔2nh3(g) at equilibrium, the concentration of nh3,h2andn2 are 1.2×10−2,3.0×10−2and1.5×10−2 respectivley. step 1: write the expression for the equilibrium constant (kc). step 2: substitute the equilibrium concentrations into the kc expression. step 3: calculate the numerator. step 4: calculate the denominator. step 5: calculate kc.
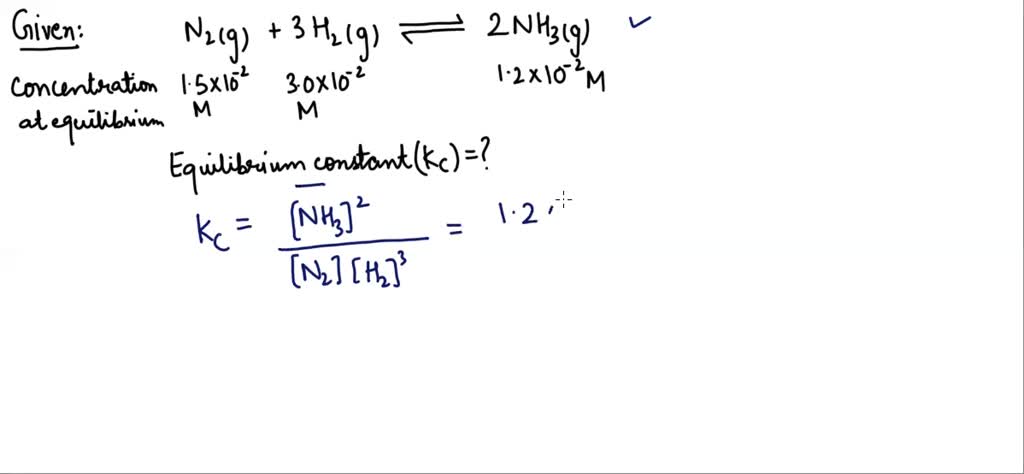
Solved The Following Concentrations Were Obtained For The Formation Calculate the equilibrium constant (kc) forthe formation of nh (3)in the following reactions. n (2) (g) 3h (2) (g) harr 2nh (3) (g) at equilibrium, the concent. We need to know two things in order to calculate the numeric value of the equilibrium constant: the balanced equation for the reaction system, including the physical states of each species. from this the equilibrium expression for calculating k c or k p is derived. Calculate the equilibrium constant kc when 0.25 moles of ethanol and ethanoic acid are mixed together with a few drops sulfuric acid catalyst in a sealed flask. the reaction is heated to a constant temperature and allowed to reach equilibrium. Question what is the value of the equilibrium constant at 500 °c for the formation of nh3 according to the following equation: an equilibrium mixture of nh3(g), h2(g), and n2(g) at 500 °c was found to contain 1.35 m h2, 1.15 m n2, and 4.12 × 10−1 m nh3. show hide answer kc = 6.00 read more ».

Solved Determine The Equilibrium Constant Kc For The Chegg Calculate the equilibrium constant kc when 0.25 moles of ethanol and ethanoic acid are mixed together with a few drops sulfuric acid catalyst in a sealed flask. the reaction is heated to a constant temperature and allowed to reach equilibrium. Question what is the value of the equilibrium constant at 500 °c for the formation of nh3 according to the following equation: an equilibrium mixture of nh3(g), h2(g), and n2(g) at 500 °c was found to contain 1.35 m h2, 1.15 m n2, and 4.12 × 10−1 m nh3. show hide answer kc = 6.00 read more ». To know the relationship between the equilibrium constant and the rate constants for the forward and reverse reactions. to write an equilibrium constant expression for any reaction. Example #1: calculate the equilibrium constant (k c) for the following reaction: 1) the first thing to do is write the equilibrium expression for the reaction as written in the problem. this is what to write: 2) now, all you have to do is substitute numbers into the equilibrium expression:. With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium constant with ease! where [a] and [b] are the molar concentrations of the reactants, and [c] and [d] are the molar concentrations of the products. Calculate the equilibrium constant (kc) for the formation of nh3 in the following reaction: n2( g) 3h2( g) ⇌2nh3( g) at equilibrium, the concentration of nh3,h2 and n2 are 1.2×10−2,3.0×10−2 and 1.5×10−2m respectively.

Solved The Equilibrium Constant Kc For The Following Reaction Is 16 To know the relationship between the equilibrium constant and the rate constants for the forward and reverse reactions. to write an equilibrium constant expression for any reaction. Example #1: calculate the equilibrium constant (k c) for the following reaction: 1) the first thing to do is write the equilibrium expression for the reaction as written in the problem. this is what to write: 2) now, all you have to do is substitute numbers into the equilibrium expression:. With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium constant with ease! where [a] and [b] are the molar concentrations of the reactants, and [c] and [d] are the molar concentrations of the products. Calculate the equilibrium constant (kc) for the formation of nh3 in the following reaction: n2( g) 3h2( g) ⇌2nh3( g) at equilibrium, the concentration of nh3,h2 and n2 are 1.2×10−2,3.0×10−2 and 1.5×10−2m respectively.
Comments are closed.