Acid File Formats Writing Apache Iceberg On Waitingforcode
Acid File Formats Writing Apache Iceberg On Waitingforcode An acid is any substance that in water solution tastes sour, changes blue litmus paper to red, reacts with some metals to liberate hydrogen, reacts with bases to form salts, and promotes chemical reactions (acid catalysis). In chemistry, an acid is a chemical species that donates hydrogen ions or protons or accepts an electron pair. acids react with bases and some metals via a neutralization reaction that forms a salt.
Acid File Formats Writing Apache Iceberg On Waitingforcode An acid is a chemical that gives away protons or accepts electrons, like vinegar or lemons. there are different kinds of acids, like arrhenius, brønsted lowry, and lewis acids. There are two main definitions of acid used by chemists today. a brønsted–lowry acid is a chemical that can donate a hydrogen ion (h ) (generally speaking, this will be a proton) to another substance. a lewis acid is a chemical that can receive a pair of electrons from another substance. The meaning of acid is a sour substance; specifically : any of various typically water soluble and sour compounds that in solution are capable of reacting with a base to form a salt, redden litmus, and have a ph less than 7, that are hydrogen containing molecules or ions able to give up a proton to a base, or that are substances able to accept. An acid is a substance that forms hydrogen ions h when dissolved in water, and a base is a substance that forms hydroxide ions oh when dissolved in water. for example, hydrochloric acid (hcl hcl) is an acid because it forms h h when it dissolves in water. hcl(g) water h (aq) cl−(aq) h c l (g) water h (a q) c l (a q).
Table File Formats Compaction Apache Iceberg On Waitingforcode The meaning of acid is a sour substance; specifically : any of various typically water soluble and sour compounds that in solution are capable of reacting with a base to form a salt, redden litmus, and have a ph less than 7, that are hydrogen containing molecules or ions able to give up a proton to a base, or that are substances able to accept. An acid is a substance that forms hydrogen ions h when dissolved in water, and a base is a substance that forms hydroxide ions oh when dissolved in water. for example, hydrochloric acid (hcl hcl) is an acid because it forms h h when it dissolves in water. hcl(g) water h (aq) cl−(aq) h c l (g) water h (a q) c l (a q). Acid definition: a compound usually having a sour taste and capable of neutralizing alkalis and reddening blue litmus paper, containing hydrogen that can be replaced by a metal or an electropositive group to form a salt, or containing an atom that can accept a pair of electrons from a base. Let’s discuss the question: “what is an acid?”, and take a look at three of the most common acid definitions in chemistry! overview of acids in life, acids can be extremely variable in form and function. across all definitions, they have a few underlying characteristics, such as: they make food taste sour. In simple terms, acids are substances that taste sour and can turn blue litmus paper red, indicating their acidic nature. they’re known for their ability to react with bases to form water and salts, a fundamental reaction in chemistry. Acids are known for their sour taste, ability to turn paper red, and their ability to react with metals and carbonates. acids are both natural and man made and can be found in a variety of substances. we will look at some examples of acids and their uses.
Table File Formats Reading Path Apache Iceberg On Waitingforcode Acid definition: a compound usually having a sour taste and capable of neutralizing alkalis and reddening blue litmus paper, containing hydrogen that can be replaced by a metal or an electropositive group to form a salt, or containing an atom that can accept a pair of electrons from a base. Let’s discuss the question: “what is an acid?”, and take a look at three of the most common acid definitions in chemistry! overview of acids in life, acids can be extremely variable in form and function. across all definitions, they have a few underlying characteristics, such as: they make food taste sour. In simple terms, acids are substances that taste sour and can turn blue litmus paper red, indicating their acidic nature. they’re known for their ability to react with bases to form water and salts, a fundamental reaction in chemistry. Acids are known for their sour taste, ability to turn paper red, and their ability to react with metals and carbonates. acids are both natural and man made and can be found in a variety of substances. we will look at some examples of acids and their uses.
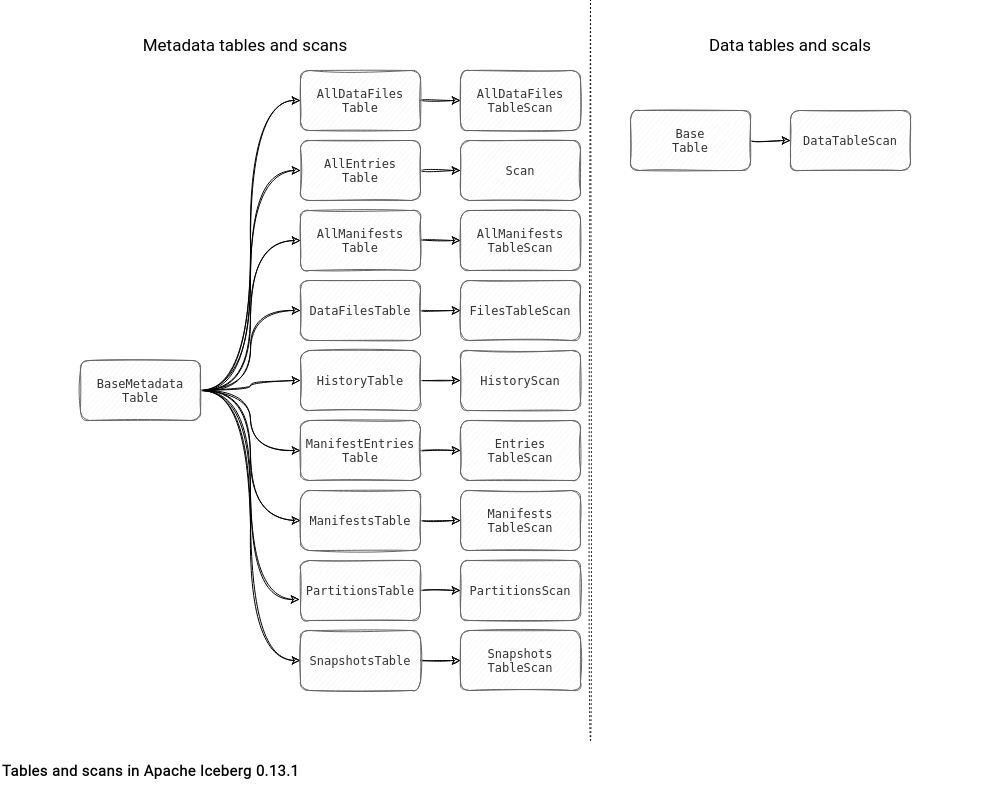
Table File Formats Reading Path Apache Iceberg On Waitingforcode In simple terms, acids are substances that taste sour and can turn blue litmus paper red, indicating their acidic nature. they’re known for their ability to react with bases to form water and salts, a fundamental reaction in chemistry. Acids are known for their sour taste, ability to turn paper red, and their ability to react with metals and carbonates. acids are both natural and man made and can be found in a variety of substances. we will look at some examples of acids and their uses.
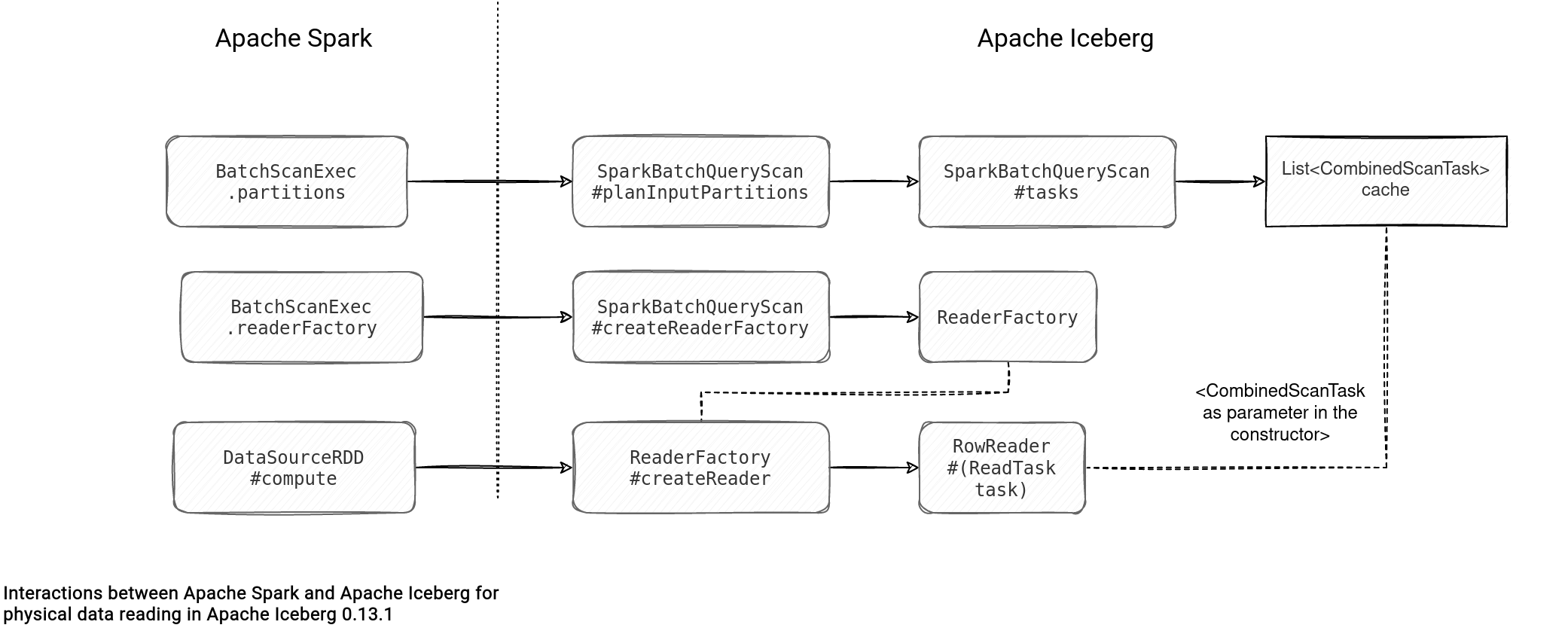
Table File Formats Reading Path Apache Iceberg On Waitingforcode
Comments are closed.